DNase I hypersensitivity and epsilon-globin transcriptional enhancement are separable in locus control region (LCR) HS1 mutant human beta-globin YAC transgenic mice
- PMID: 20231293
- PMCID: PMC2863201
- DOI: 10.1074/jbc.M110.116525
DNase I hypersensitivity and epsilon-globin transcriptional enhancement are separable in locus control region (LCR) HS1 mutant human beta-globin YAC transgenic mice
Abstract
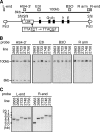
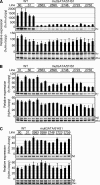
Expression of the five beta-like globin genes (epsilon, Ggamma, Agamma, delta, beta) in the human beta-globin locus depends on enhancement by the locus control region, which consists of five DNase I hypersensitive sites (5'HS1 through 5'HS5). We report here a novel enhancer activity in 5'HS1 that appears to be potent in transfected K562 cells. Deletion analyses identified a core activating element that bound to GATA-1, and a two-nucleotide mutation that disrupted GATA-1 binding in vitro abrogated 5'HS1 enhancer activity in transfection experiments. To determine the in vivo role of this GATA site, we generated multiple lines of human beta-globin YAC transgenic mice bearing the same two-nucleotide mutation. In the mutant mice, epsilon-, but not gamma-globin, gene expression in primitive erythroid cells was severely attenuated, while adult beta-globin gene expression in definitive erythroid cells was unaffected. Interestingly, DNaseI hypersensitivity near the 5'HS1 mutant sequence was eliminated in definitive erythroid cells, whereas it was only mildly affected in primitive erythroid cells. We therefore conclude that, although the GATA site in 5'HS1 is critical for efficient epsilon-globin gene expression, hypersensitive site formation per se is independent of 5'HS1 function, if any, in definitive erythroid cells.
Figures




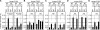

Similar articles
-
Deletion of the human beta-globin LCR 5'HS4 or 5'HS1 differentially affects beta-like globin gene expression in beta-YAC transgenic mice.Blood Cells Mol Dis. 2007 Jul-Aug;39(1):44-55. doi: 10.1016/j.bcmd.2007.02.006. Epub 2007 Apr 11. Blood Cells Mol Dis. 2007. PMID: 17433733 Free PMC article.
-
Full activity from human beta-globin locus control region transgenes requires 5'HS1, distal beta-globin promoter, and 3' beta-globin sequences.Blood. 1998 Jul 15;92(2):653-63. Blood. 1998. PMID: 9657768
-
Targeted deletion of 5'HS2 enhancer of β-globin locus control region in K562 cells.Yi Chuan. 2022 Sep 20;44(9):783-797. doi: 10.16288/j.yczz.22-201. Yi Chuan. 2022. PMID: 36384955
-
beta-YAC transgenic mice for studying LCR function.Ann N Y Acad Sci. 1998 Jun 30;850:28-37. doi: 10.1111/j.1749-6632.1998.tb10459.x. Ann N Y Acad Sci. 1998. PMID: 9668524 Review.
-
Chromatin loop formation in the β-globin locus and its role in globin gene transcription.Mol Cells. 2012 Jul;34(1):1-5. doi: 10.1007/s10059-012-0048-8. Epub 2012 May 18. Mol Cells. 2012. PMID: 22610406 Free PMC article. Review.
Cited by
-
Shift in GATA3 functions, and GATA3 mutations, control progression and clinical presentation in breast cancer.Breast Cancer Res. 2014 Nov 20;16(6):464. doi: 10.1186/s13058-014-0464-0. Breast Cancer Res. 2014. PMID: 25410484 Free PMC article.
-
The Drosophila Zinc Finger Transcription Factor Ouija Board Controls Ecdysteroid Biosynthesis through Specific Regulation of spookier.PLoS Genet. 2015 Dec 10;11(12):e1005712. doi: 10.1371/journal.pgen.1005712. eCollection 2015 Dec. PLoS Genet. 2015. PMID: 26658797 Free PMC article.
-
LCR 5' hypersensitive site specificity for globin gene activation within the active chromatin hub.Nucleic Acids Res. 2012 Dec;40(22):11256-69. doi: 10.1093/nar/gks900. Epub 2012 Oct 4. Nucleic Acids Res. 2012. PMID: 23042246 Free PMC article.
-
Transcriptional activation of the mouse Scd2 gene by interdependent enhancers and long noncoding RNAs in ovarian granulosa cells.J Reprod Dev. 2020 Oct 13;66(5):435-444. doi: 10.1262/jrd.2019-161. Epub 2020 Jun 5. J Reprod Dev. 2020. PMID: 32507774 Free PMC article.
-
Identifying and mapping cell-type-specific chromatin programming of gene expression.Proc Natl Acad Sci U S A. 2014 Feb 11;111(6):E645-54. doi: 10.1073/pnas.1312523111. Epub 2014 Jan 27. Proc Natl Acad Sci U S A. 2014. PMID: 24469817 Free PMC article.
References
-
- Stamatoyannopoulos G., Neinhuis A. W. (1994) in The Molecular Basis of Blood Diseases (Stamatoyannopoulos G., Neinhuis A. W., Majerus P., Varmus H. ed), pp. 107–155, 2 Ed., W. B. Saunders Co., New York
-
- Grosveld F., Greaves D., Philipsen S., Talbot D., Pruzina S., deBoer E., Hanscombe O., Belhumeur P., Hurst J., Fraser P., et al. (1990) Ann. N.Y. Acad. Sci. 612, 152–159 - PubMed
-
- Strouboulis J., Dillon N., Grosveld F. (1992) Genes Dev. 6, 1857–1864 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

