Phosphoinositide 3-kinase pathway activation in phosphate and tensin homolog (PTEN)-deficient prostate cancer cells is independent of receptor tyrosine kinases and mediated by the p110beta and p110delta catalytic subunits
- PMID: 20231295
- PMCID: PMC2865293
- DOI: 10.1074/jbc.M109.085696
Phosphoinositide 3-kinase pathway activation in phosphate and tensin homolog (PTEN)-deficient prostate cancer cells is independent of receptor tyrosine kinases and mediated by the p110beta and p110delta catalytic subunits
Abstract
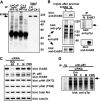
Class IA phosphoinositide 3-kinase (PI3K) p110 catalytic subunits are activated upon Src homology 2 domain-mediated binding of their p85 regulatory subunits to tyrosine-phosphorylated pYXXM motifs in receptor tyrosine kinases (RTKs) or adaptor proteins. The PI3K pathway is activated by phosphate and tensin homolog (PTEN) loss in most prostate cancers (PCa), but the contribution of upstream RTKs that may be targeted therapeutically has not been assessed. Immunoblotting of p85-associated proteins in serum-starved PTEN-deficient LNCaP and C4-2 PCa cells showed a small set of discrete tyrosine-phosphorylated proteins, but these proteins were not recognized by an anti-pYXXM motif antibody and were not found in PTEN-deficient PC3 PCa cells. LC/MS/MS using label-free proteomics and immunoblotting showed that p85 was associated primarily with p110beta and p110delta. An interaction with ErbB3 was also detected but was independent of ErbB3 tyrosine phosphorylation and was not required for basal PI3K activity. Basal tyrosine phosphorylation of p110beta and p110delta could be blocked by c-Src inhibitors, but this did not suppress PI3K activity, which was similarly independent of Ras. Basal PI3K activity was mediated by p110beta in PC3 cells and by both p110beta and p110delta in LNCaP cells, whereas p110alpha was required for PI3K activation in response to RTK stimulation by heregulin-beta1. These findings show that basal PI3K activity in PTEN-deficient PCa cells is RTK-independent and can be mediated by p110beta and p110delta. Increased p110beta expression in PCa may be required for RTK-independent PI3K pathway activation in adult prostate epithelium with genetic or epigenetic PTEN down-regulation.
Figures
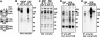






Similar articles
-
Both p110α and p110β isoforms of PI3K can modulate the impact of loss-of-function of the PTEN tumour suppressor.Biochem J. 2012 Feb 15;442(1):151-9. doi: 10.1042/BJ20111741. Biochem J. 2012. PMID: 22150431 Free PMC article.
-
Activation of nonreceptor tyrosine kinase Bmx/Etk mediated by phosphoinositide 3-kinase, epidermal growth factor receptor, and ErbB3 in prostate cancer cells.J Biol Chem. 2007 Nov 9;282(45):32689-98. doi: 10.1074/jbc.M703412200. Epub 2007 Sep 6. J Biol Chem. 2007. PMID: 17823122
-
Should individual PI3 kinase isoforms be targeted in cancer?Curr Opin Cell Biol. 2009 Apr;21(2):199-208. doi: 10.1016/j.ceb.2008.12.007. Epub 2009 Feb 4. Curr Opin Cell Biol. 2009. PMID: 19200708 Free PMC article. Review.
-
CRKL Mediates p110β-Dependent PI3K Signaling in PTEN-Deficient Cancer Cells.Cell Rep. 2017 Jul 18;20(3):549-557. doi: 10.1016/j.celrep.2017.06.054. Cell Rep. 2017. PMID: 28723560 Free PMC article.
-
Class I phosphoinositide 3-kinase (PI3K) regulatory subunits and their roles in signaling and disease.Adv Biol Regul. 2020 Jan;75:100657. doi: 10.1016/j.jbior.2019.100657. Epub 2019 Sep 28. Adv Biol Regul. 2020. PMID: 31611073 Review.
Cited by
-
Mechanisms of resistance in castration-resistant prostate cancer (CRPC).Transl Androl Urol. 2015 Jun;4(3):365-80. doi: 10.3978/j.issn.2223-4683.2015.05.02. Transl Androl Urol. 2015. PMID: 26814148 Free PMC article. Review.
-
Characterization of a novel p110β-specific inhibitor BL140 that overcomes MDV3100-resistance in castration-resistant prostate cancer cells.Prostate. 2017 Aug;77(11):1187-1198. doi: 10.1002/pros.23377. Epub 2017 Jun 20. Prostate. 2017. PMID: 28631436 Free PMC article.
-
PI3King the right partner: unique interactions and signaling by p110β.Postdoc J. 2015 Jun;3(6):71-87. doi: 10.14304/surya.jpr.v3n6.8. Postdoc J. 2015. PMID: 26140278 Free PMC article.
-
PI3Kβ-A Versatile Transducer for GPCR, RTK, and Small GTPase Signaling.Endocrinology. 2019 Mar 1;160(3):536-555. doi: 10.1210/en.2018-00843. Endocrinology. 2019. PMID: 30601996 Free PMC article. Review.
-
Inhibition of PTEN activity aggravates cisplatin-induced acute kidney injury.Oncotarget. 2017 Sep 8;8(61):103154-103166. doi: 10.18632/oncotarget.20790. eCollection 2017 Nov 28. Oncotarget. 2017. PMID: 29262553 Free PMC article.
References
-
- Engelman J. A., Luo J., Cantley L. C. (2006) Nat. Rev. Genet. 7, 606–619 - PubMed
-
- Songyang Z., Shoelson S. E., Chaudhuri M., Gish G., Pawson T., Haser W. G., King F., Roberts T., Ratnofsky S., Lechleider R. J. (1993) Cell 72, 767–778 - PubMed
-
- Carracedo A., Pandolfi P. P. (2008) Oncogene 27, 5527–5541 - PubMed
-
- Keniry M., Parsons R. (2008) Oncogene 27, 5477–5485 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

