Functional interaction between telomere protein TPP1 and telomerase
- PMID: 20231318
- PMCID: PMC2841338
- DOI: 10.1101/gad.1881810
Functional interaction between telomere protein TPP1 and telomerase
Abstract
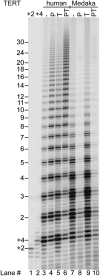
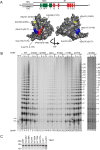
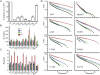
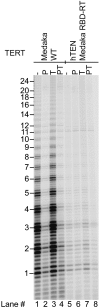
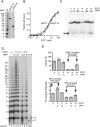
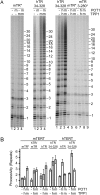
Human chromosome end-capping and telomerase regulation require POT1 (Protection of Telomeres 1) and TPP1 proteins, which bind to the 3' ssDNA extension of human telomeres. POT1-TPP1 binding to telomeric DNA activates telomerase repeat addition processivity. We now provide evidence that this POT1-TPP1 activation requires specific interactions with telomerase, rather than it being a DNA substrate-specific effect. First, telomerase from the fish medaka, which extends the same telomeric DNA primer as human telomerase, was not activated by human POT1-TPP1. Second, mutation of a conserved glycine, Gly100 in the TEN (telomerase essential N-terminal) domain of TERT, abolished the enhancement of telomerase processivity by POT1-TPP1, in contrast to other single amino acid mutations. Chimeric human-fish telomerases that contained the human TEN domain were active but not stimulated by POT1-TPP1, showing that additional determinants of processivity lie outside the TEN domain. Finally, primers bound to mouse POT1A and human TPP1 were activated for extension by human telomerase, whereas mPOT1A-mTPP1 was most active with mouse telomerase, indicating that these mammalian telomerases have specificity for their respective TPP1 proteins. We suggest that a sequence-specific interaction between TPP1 in the TPP1-POT1-telomeric DNA complex and the G100 region of the TEN domain of TERT is necessary for high-processivity telomerase action.
Figures
Similar articles
-
POT1-TPP1 enhances telomerase processivity by slowing primer dissociation and aiding translocation.EMBO J. 2010 Mar 3;29(5):924-33. doi: 10.1038/emboj.2009.409. Epub 2010 Jan 21. EMBO J. 2010. PMID: 20094033 Free PMC article.
-
The TEL patch of telomere protein TPP1 mediates telomerase recruitment and processivity.Nature. 2012 Dec 13;492(7428):285-9. doi: 10.1038/nature11648. Epub 2012 Oct 24. Nature. 2012. PMID: 23103865 Free PMC article.
-
The POT1-TPP1 telomere complex is a telomerase processivity factor.Nature. 2007 Feb 1;445(7127):506-10. doi: 10.1038/nature05454. Epub 2007 Jan 21. Nature. 2007. PMID: 17237768
-
Structural biology of telomeres and telomerase.Cell Mol Life Sci. 2020 Jan;77(1):61-79. doi: 10.1007/s00018-019-03369-x. Epub 2019 Nov 14. Cell Mol Life Sci. 2020. PMID: 31728577 Free PMC article. Review.
-
POT1-TPP1 telomere length regulation and disease.Comput Struct Biotechnol J. 2020 Jul 3;18:1939-1946. doi: 10.1016/j.csbj.2020.06.040. eCollection 2020. Comput Struct Biotechnol J. 2020. PMID: 32774788 Free PMC article. Review.
Cited by
-
Human telomerase: biogenesis, trafficking, recruitment, and activation.Genes Dev. 2015 Jun 1;29(11):1095-105. doi: 10.1101/gad.263863.115. Genes Dev. 2015. PMID: 26063571 Free PMC article. Review.
-
Evolution of CST function in telomere maintenance.Cell Cycle. 2010 Aug 15;9(16):3157-65. doi: 10.4161/cc.9.16.12547. Epub 2010 Aug 26. Cell Cycle. 2010. PMID: 20697207 Free PMC article. Review.
-
Direct observation of nucleic acid binding dynamics by the telomerase essential N-terminal domain.Nucleic Acids Res. 2018 Apr 6;46(6):3088-3102. doi: 10.1093/nar/gky117. Nucleic Acids Res. 2018. PMID: 29474579 Free PMC article.
-
Telomerase enzymatic component hTERT shortens long telomeres in human cells.Cell Cycle. 2014;13(11):1765-76. doi: 10.4161/cc.28705. Epub 2014 Apr 10. Cell Cycle. 2014. PMID: 24721976 Free PMC article.
-
Identification of human TERT elements necessary for telomerase recruitment to telomeres.Elife. 2014 Oct 1;3:e03563. doi: 10.7554/eLife.03563. Elife. 2014. PMID: 25271372 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
