Joubert syndrome Arl13b functions at ciliary membranes and stabilizes protein transport in Caenorhabditis elegans
- PMID: 20231383
- PMCID: PMC2845074
- DOI: 10.1083/jcb.200908133
Joubert syndrome Arl13b functions at ciliary membranes and stabilizes protein transport in Caenorhabditis elegans
Erratum in
- J Cell Biol. 2010 Apr 5;189(1):187
Abstract
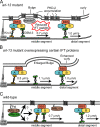
The small ciliary G protein Arl13b is required for cilium biogenesis and sonic hedgehog signaling and is mutated in patients with Joubert syndrome (JS). In this study, using Caenorhabditis elegans and mammalian cell culture systems, we investigated the poorly understood ciliary and molecular basis of Arl13b function. First, we show that Arl13b/ARL-13 localization is frequently restricted to a proximal ciliary compartment, where it associates with ciliary membranes via palmitoylation modification motifs. Next, we find that loss-of-function C. elegans arl-13 mutants possess defects in cilium morphology and ultrastructure, as well as defects in ciliary protein localization and transport; ciliary transmembrane proteins abnormally accumulate, PKD-2 ciliary abundance is elevated, and anterograde intraflagellar transport (IFT) is destabilized. Finally, we show that arl-13 interacts genetically with other ciliogenic and ciliary transport-associated genes in maintaining cilium structure/morphology and anterograde IFT stability. Together, these data implicate a role for JS-associated Arl13b at ciliary membranes, where it regulates ciliary transmembrane protein localizations and anterograde IFT assembly stability.
Figures



References
-
- Blacque O.E., Reardon M.J., Li C., McCarthy J., Mahjoub M.R., Ansley S.J., Badano J.L., Mah A.K., Beales P.L., Davidson W.S., et al. 2004. Loss of C. elegans BBS-7 and BBS-8 protein function results in cilia defects and compromised intraflagellar transport. Genes Dev. 18:1630–1642 10.1101/gad.1194004 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

