Focal adhesions are sites of integrin extension
- PMID: 20231384
- PMCID: PMC2845069
- DOI: 10.1083/jcb.200907174
Focal adhesions are sites of integrin extension
Abstract
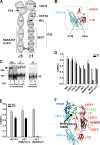
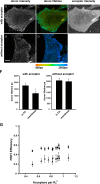
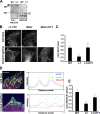
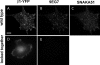
Integrins undergo global conformational changes that specify their activation state. Current models portray the inactive receptor in a bent conformation that upon activation converts to a fully extended form in which the integrin subunit leg regions are separated to enable ligand binding and subsequent signaling. To test the applicability of this model in adherent cells, we used a fluorescent resonance energy transfer (FRET)-based approach, in combination with engineered integrin mutants and monoclonal antibody reporters, to image integrin alpha5beta1 conformation. We find that restricting leg separation causes the integrin to adopt a bent conformation that is unable to respond to agonists and mediate cell spreading. By measuring FRET between labeled alpha5beta1 and the cell membrane, we find extended receptors are enriched in focal adhesions compared with adjacent regions of the plasma membrane. These results demonstrate definitely that major quaternary rearrangements of beta1-integrin subunits occur in adherent cells and that conversion from a bent to extended form takes place at focal adhesions.
Figures

References
-
- Amiot M., Bernard A., Tran H.C., Leca G., Kanellopoulos J.M., Boumsell L. 1986. The human cell surface glycoprotein complex (gp 120,200) recognized by monoclonal antibody K20 is a component binding to phytohaemagglutinin on T cells. Scand. J. Immunol. 23:109–118 10.1111/j.1365-3083.1986.tb01948.x - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

