Azithromycin alters macrophage phenotype and pulmonary compartmentalization during lung infection with Pseudomonas
- PMID: 20231397
- PMCID: PMC2876369
- DOI: 10.1128/AAC.01424-09
Azithromycin alters macrophage phenotype and pulmonary compartmentalization during lung infection with Pseudomonas
Abstract
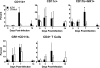
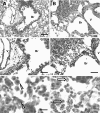
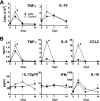
Infection with mucoid strains of Pseudomonas aeruginosa in chronic inflammatory diseases of the airway is difficult to eradicate and can cause excessive inflammation. The roles of alternatively activated and regulatory subsets of macrophages in this pathophysiological process are not well characterized. We previously demonstrated that azithromycin induces an alternatively activated macrophage-like phenotype in vitro. In the present study, we tested whether azithromycin affects the macrophage activation status and migration in the lungs of P. aeruginosa-infected mice. C57BL/6 mice received daily doses of oral azithromycin and were infected intratracheally with a mucoid strain of P. aeruginosa. The properties of macrophage activation, immune cell infiltration, and markers of pulmonary inflammation in the lung interstitial and alveolar compartments were evaluated postinfection. Markers of alternative macrophage activation were induced by azithromycin treatment, including the surface expression of the mannose receptor, the upregulation of arginase 1, and a decrease in the production of proinflammatory cytokines. Additionally, azithromycin increased the number of CD11b(+) monocytes and CD4(+) T cells that infiltrated the alveolar compartment. A predominant subset of CD11b(+) cells was Gr-1 positive (Gr-1(+)), indicative of a subset of cells that has been shown to be immunoregulatory. These differences corresponded to decreases in neutrophil influx into the lung parenchyma and alteration of the characteristics of peribronchiolar inflammation without any change in the clearance of the organism. These results suggest that the immunomodulatory effects of azithromycin are associated with the induction of alternative and regulatory macrophage activation characteristics and alteration of cellular compartmentalization during infection.
Figures

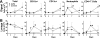



Similar articles
-
Myeloid arginase-1 controls excessive inflammation and modulates T cell responses in Pseudomonas aeruginosa pneumonia.Immunobiology. 2021 Jan;226(1):152034. doi: 10.1016/j.imbio.2020.152034. Epub 2020 Nov 24. Immunobiology. 2021. PMID: 33278710 Free PMC article.
-
Azithromycin reduces exaggerated cytokine production by M1 alveolar macrophages in cystic fibrosis.Am J Respir Cell Mol Biol. 2009 Nov;41(5):590-602. doi: 10.1165/rcmb.2008-0155OC. Epub 2009 Feb 24. Am J Respir Cell Mol Biol. 2009. PMID: 19244203
-
Azithromycin blocks neutrophil recruitment in Pseudomonas endobronchial infection.Am J Respir Crit Care Med. 2004 Dec 15;170(12):1331-9. doi: 10.1164/rccm.200402-200OC. Epub 2004 Sep 10. Am J Respir Crit Care Med. 2004. PMID: 15361366
-
Airway immunometabolites fuel Pseudomonas aeruginosa infection.Respir Res. 2020 Dec 10;21(1):326. doi: 10.1186/s12931-020-01591-x. Respir Res. 2020. PMID: 33302964 Free PMC article. Review.
-
Immunomodulatory indications of azithromycin in respiratory disease: a concise review for the clinician.Postgrad Med. 2017 Jun;129(5):493-499. doi: 10.1080/00325481.2017.1285677. Epub 2017 Feb 1. Postgrad Med. 2017. PMID: 28116959 Review.
Cited by
-
Obesity increases airway hyperresponsiveness via the TNF-α pathway and treating obesity induces recovery.PLoS One. 2015 Feb 6;10(2):e0116540. doi: 10.1371/journal.pone.0116540. eCollection 2015. PLoS One. 2015. PMID: 25658739 Free PMC article.
-
Can endolysosomal deacidification and inhibition of autophagy prevent severe COVID-19?Life Sci. 2020 Dec 1;262:118541. doi: 10.1016/j.lfs.2020.118541. Epub 2020 Oct 6. Life Sci. 2020. PMID: 33035581 Free PMC article. Review.
-
Delayed Azithromycin Treatment Improves Recovery After Mouse Spinal Cord Injury.Front Cell Neurosci. 2019 Nov 6;13:490. doi: 10.3389/fncel.2019.00490. eCollection 2019. Front Cell Neurosci. 2019. PMID: 31780896 Free PMC article.
-
Predictive screening of M1 and M2 macrophages reveals the immunomodulatory effectiveness of post spinal cord injury azithromycin treatment.Sci Rep. 2017 Jan 6;7:40144. doi: 10.1038/srep40144. Sci Rep. 2017. PMID: 28057928 Free PMC article.
-
Cytokine mediated tissue fibrosis.Biochim Biophys Acta. 2013 Jul;1832(7):1049-60. doi: 10.1016/j.bbadis.2012.09.014. Epub 2012 Oct 6. Biochim Biophys Acta. 2013. PMID: 23046809 Free PMC article. Review.
References
-
- Aghai, Z. H., A. Kode, J. G. Saslow, T. Nakhla, S. Farhath, G. E. Stahl, R. Eydelman, L. Strande, P. Leone, and I. Rahman. 2007. Azithromycin suppresses activation of nuclear factor-kappa B and synthesis of pro-inflammatory cytokines in tracheal aspirate cells from premature infants. Pediatr. Res. 62:483-488. - PubMed
-
- Barnes, P. 2008. Immunology of asthma and chronic obstructive pulmonary disease. Nat. Rev. Immunol. 8:183-192. - PubMed
-
- Benoit, M., B. Desnues, and J. L. Mege. 2008. Macrophage polarization in bacterial infections. J. Immunol. 181:3733-3739. - PubMed
-
- Bonfield, T., M. W. Konstan, and M. Berger. 1999. Altered respiratory epithelial cell cytokine production in cystic fibrosis. J. Allergy Clin. Immunol. 104:72-78. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

