Regulation of virulence gene transcripts by the Francisella novicida orphan response regulator PmrA: role of phosphorylation and evidence of MglA/SspA interaction
- PMID: 20231408
- PMCID: PMC2863534
- DOI: 10.1128/IAI.00021-10
Regulation of virulence gene transcripts by the Francisella novicida orphan response regulator PmrA: role of phosphorylation and evidence of MglA/SspA interaction
Abstract
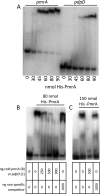
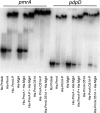
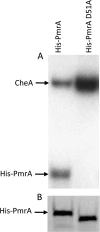
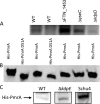
Francisella tularensis subsp. tularensis is the etiologic agent of tularemia and has been designated a category A biothreat agent by the CDC. Tularemia is characterized by replication and dissemination within host phagocytes. Intramacrophage growth is dependent upon the regulation of Francisella pathogenicity island (FPI) virulence genes, which is poorly understood. Two-component regulatory systems (TCS) are widely employed by Gram-negative bacteria to monitor and respond to environmental signals. Virulent strains of F. tularensis subsp. tularensis are devoid of classical, tandemly arranged TCS genes, but orphaned members, such as that encoding the response regulator PmrA, have been identified. In the F. novicida model system, previous work has shown that a pmrA mutant shows decreased expression of FPI genes, is deficient for intramacrophage growth, and is avirulent in the mouse model. Here, we determine that phosphorylation aids PmrA binding to regulated promoters pmrA and the FPI-encoded pdpD, and KdpD is the histidine kinase primarily responsible for phosphorylation of PmrA at the aspartic acid at position 51 (D51). A strain expressing PmrA D51A retains some DNA binding but exhibits reduced expression of the PmrA regulon, is deficient for intramacrophage replication, and is attenuated in the mouse model. With regard to virulence gene induction, PmrA coprecipitates with the FPI transcription factors MglA and SspA, which bind RNA polymerase. Together, these data suggest a model of Francisella gene regulation that includes a TCS consisting of KdpD and PmrA. Once phosphorylated, PmrA binds to regulated gene promoters recruiting free or RNA polymerase-bound MglA and SspA to initiate FPI gene transcription.
Figures
Similar articles
-
The Sensor Kinase QseC Regulates the Unlinked PmrA Response Regulator and Downstream Gene Expression in Francisella.J Bacteriol. 2020 Oct 8;202(21):e00321-20. doi: 10.1128/JB.00321-20. Print 2020 Oct 8. J Bacteriol. 2020. PMID: 32839173 Free PMC article.
-
Identification of an orphan response regulator required for the virulence of Francisella spp. and transcription of pathogenicity island genes.Infect Immun. 2007 Jul;75(7):3305-14. doi: 10.1128/IAI.00351-07. Epub 2007 Apr 23. Infect Immun. 2007. PMID: 17452468 Free PMC article.
-
Identification of fevR, a novel regulator of virulence gene expression in Francisella novicida.Infect Immun. 2008 Aug;76(8):3473-80. doi: 10.1128/IAI.00430-08. Epub 2008 Jun 16. Infect Immun. 2008. PMID: 18559431 Free PMC article.
-
Molecular and genetic basis of pathogenesis in Francisella tularensis.Ann N Y Acad Sci. 2007 Jun;1105:138-59. doi: 10.1196/annals.1409.010. Epub 2007 Mar 29. Ann N Y Acad Sci. 2007. PMID: 17395737 Review.
-
Two-Component Systems in Francisella Species.Front Cell Infect Microbiol. 2019 Jun 12;9:198. doi: 10.3389/fcimb.2019.00198. eCollection 2019. Front Cell Infect Microbiol. 2019. PMID: 31263682 Free PMC article. Review.
Cited by
-
Screen of FDA-approved drug library identifies maprotiline, an antibiofilm and antivirulence compound with QseC sensor-kinase dependent activity in Francisella novicida.Virulence. 2015;6(5):487-503. doi: 10.1080/21505594.2015.1046029. Virulence. 2015. PMID: 26155740 Free PMC article.
-
Elucidation of a mechanism of oxidative stress regulation in Francisella tularensis live vaccine strain.Mol Microbiol. 2016 Sep;101(5):856-78. doi: 10.1111/mmi.13426. Epub 2016 Jun 16. Mol Microbiol. 2016. PMID: 27205902 Free PMC article.
-
The Role of the Francisella Tularensis Pathogenicity Island in Type VI Secretion, Intracellular Survival, and Modulation of Host Cell Signaling.Front Microbiol. 2010 Dec 21;1:136. doi: 10.3389/fmicb.2010.00136. eCollection 2010. Front Microbiol. 2010. PMID: 21687753 Free PMC article.
-
From the Outside-In: The Francisella tularensis Envelope and Virulence.Front Cell Infect Microbiol. 2015 Dec 23;5:94. doi: 10.3389/fcimb.2015.00094. eCollection 2015. Front Cell Infect Microbiol. 2015. PMID: 26779445 Free PMC article. Review.
-
Beyond Homeostasis: Potassium and Pathogenesis during Bacterial Infections.Infect Immun. 2021 Jun 16;89(7):e0076620. doi: 10.1128/IAI.00766-20. Epub 2021 Jun 16. Infect Immun. 2021. PMID: 33875474 Free PMC article. Review.
References
-
- Baron, G. S., and F. E. Nano. 1998. MglA and MglB are required for the intramacrophage growth of Francisella novicida. Mol. Microbiol. 29:247-259. - PubMed
-
- Buchan, B. W., R. L. McCaffrey, S. R. Lindemann, L. A. Allen, and B. D. Jones. 2009. Identification of migR, a regulatory element of the Francisella tularensis live vaccine strain iglABCD virulence operon required for normal replication and trafficking in macrophages. Infect. Immun. 77:2517-2529. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

