Plasmodium vivax clinical malaria is commonly observed in Duffy-negative Malagasy people
- PMID: 20231434
- PMCID: PMC2851935
- DOI: 10.1073/pnas.0912496107
Plasmodium vivax clinical malaria is commonly observed in Duffy-negative Malagasy people
Abstract
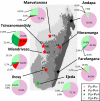
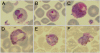
Malaria therapy, experimental, and epidemiological studies have shown that erythrocyte Duffy blood group-negative people, largely of African ancestry, are resistant to erythrocyte Plasmodium vivax infection. These findings established a paradigm that the Duffy antigen is required for P. vivax erythrocyte invasion. P. vivax is endemic in Madagascar, where admixture of Duffy-negative and Duffy-positive populations of diverse ethnic backgrounds has occurred over 2 millennia. There, we investigated susceptibility to P. vivax blood-stage infection and disease in association with Duffy blood group polymorphism. Duffy blood group genotyping identified 72% Duffy-negative individuals (FY*B(ES)/*B(ES)) in community surveys conducted at eight sentinel sites. Flow cytometry and adsorption-elution results confirmed the absence of Duffy antigen expression on Duffy-negative erythrocytes. P. vivax PCR positivity was observed in 8.8% (42/476) of asymptomatic Duffy-negative people. Clinical vivax malaria was identified in Duffy-negative subjects with nine P. vivax monoinfections and eight mixed Plasmodium species infections that included P. vivax (4.9 and 4.4% of 183 participants, respectively). Microscopy examination of blood smears confirmed blood-stage development of P. vivax, including gametocytes. Genotyping of polymorphic surface and microsatellite markers suggested that multiple P. vivax strains were infecting Duffy-negative people. In Madagascar, P. vivax has broken through its dependence on the Duffy antigen for establishing human blood-stage infection and disease. Further studies are necessary to identify the parasite and host molecules that enable this Duffy-independent P. vivax invasion of human erythrocytes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Boyd MF, Stratman-Thomas WK. Studies on benign tertian malaria. 4. On the refractoriness of negroes to inoculation with Plasmodium vivax. Am J Hyg. 1933;18:485–489.
-
- Bray RS. The susceptibility of Liberians to the Madagascar strain of Plasmodium vivax. J Parasitol. 1958;44(4, Sect 1):371–373. - PubMed
-
- O’Leary PA. Treatment of neurosyphilis by malaria: Report on the three years’ observation of the first one hundred patients treated. J Am Med Assoc. 1927;89:95–100.
-
- Zimmerman PA. In: Infectious Disease and Host-Pathogen Evolution. Dronamraju KR, editor. New York: Cambridge Univ Press; 2004. pp. 141–172.
-
- Roychoudhury AK, Nei M. Human Polymorphic Genes: World Distribution. New York: Oxford Univ Press; 1988.
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

