Induction of TGF-beta1 and TGF-beta1-dependent predominant Th17 differentiation by group A streptococcal infection
- PMID: 20231435
- PMCID: PMC2851870
- DOI: 10.1073/pnas.0904831107
Induction of TGF-beta1 and TGF-beta1-dependent predominant Th17 differentiation by group A streptococcal infection
Abstract
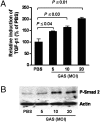
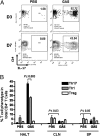
Recurrent group A Streptococcus (GAS) tonsillitis and associated autoimmune diseases indicate that the immune response to this organism can be ineffective and pathological. TGF-beta1 is recognized as an essential signal for generation of regulatory T cells (Tregs) and T helper (Th) 17 cells. Here, the impact of TGF-beta1 induction on the T-cell response in mouse nasal-associated lymphoid tissue (NALT) following intranasal (i.n.) infections is investigated. ELISA and TGF-beta1-luciferase reporter assays indicated that persistent infection of mouse NALT with GAS sets the stage for TGF-beta1 and IL-6 production, signals required for promotion of a Th17 immune response. As predicted, IL-17, the Th17 signature cytokine, was induced in a TGF-beta1 signaling-dependent manner in single-cell suspensions of both human tonsils and NALT. Intracellular cytokine staining and flow cytometry demonstrated that CD4(+) IL-17(+) T cells are the dominant T cells induced in NALT by i.n. infections. Moreover, naive mice acquired the potential to clear GAS by adoptive transfer of CD4(+) T cells from immunized IL-17A(+)/(+) mice but not cells from IL-17A(-)/(-) mice. These experiments link specific induction of TGF-beta1 by a bacterial infection to an in vivo Th17 immune response and show that this cellular response is sufficient for protection against GAS. The association of a Th17 response with GAS infection reveals a potential mechanism for destructive autoimmune responses in humans.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
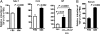
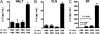
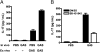
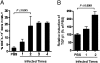

Similar articles
-
Primary induction of CD4 T cell responses in nasal associated lymphoid tissue during group A streptococcal infection.Eur J Immunol. 2004 Oct;34(10):2843-53. doi: 10.1002/eji.200425242. Eur J Immunol. 2004. PMID: 15368301
-
IL-17A-producing gammadeltaT cells promote CTL responses against Listeria monocytogenes infection by enhancing dendritic cell cross-presentation.J Immunol. 2010 Nov 15;185(10):5879-87. doi: 10.4049/jimmunol.1001763. Epub 2010 Oct 18. J Immunol. 2010. PMID: 20956351
-
Inflammasome and Fas-Mediated IL-1β Contributes to Th17/Th1 Cell Induction in Pathogenic Bacterial Infection In Vivo.J Immunol. 2017 Aug 1;199(3):1122-1130. doi: 10.4049/jimmunol.1601373. Epub 2017 Jul 3. J Immunol. 2017. PMID: 28674179
-
Th17 cell differentiation: the long and winding road.Immunity. 2008 Apr;28(4):445-53. doi: 10.1016/j.immuni.2008.03.001. Immunity. 2008. PMID: 18400187 Review.
-
[Research progress on Th17 cells].Zhejiang Da Xue Xue Bao Yi Xue Ban. 2007 Nov;36(6):620-5. doi: 10.3785/j.issn.1008-9292.2007.06.018. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2007. PMID: 18067239 Review. Chinese.
Cited by
-
Sortase A induces Th17-mediated and antibody-independent immunity to heterologous serotypes of group A streptococci.PLoS One. 2014 Sep 18;9(9):e107638. doi: 10.1371/journal.pone.0107638. eCollection 2014. PLoS One. 2014. PMID: 25232948 Free PMC article.
-
The Genetic Control of the Rheumatic Heart: Closing the Genotype-Phenotype Gap.Front Med (Lausanne). 2021 Mar 24;8:611036. doi: 10.3389/fmed.2021.611036. eCollection 2021. Front Med (Lausanne). 2021. PMID: 33842495 Free PMC article. Review.
-
"High Treg" Inflammations Promote (Most) Non-Hematologic Cancers While "Low Treg" Inflammations Promote Lymphoid Cancers.J Inflamm Res. 2020 May 21;13:209-221. doi: 10.2147/JIR.S249384. eCollection 2020. J Inflamm Res. 2020. PMID: 32547153 Free PMC article.
-
Local Th17/IgA immunity correlate with protection against intranasal infection with Streptococcus pyogenes.PLoS One. 2017 Apr 17;12(4):e0175707. doi: 10.1371/journal.pone.0175707. eCollection 2017. PLoS One. 2017. PMID: 28414746 Free PMC article.
-
The nature of innate and adaptive interleukin-17A responses in sham or bacterial inoculation.Immunology. 2012 Jul;136(3):325-33. doi: 10.1111/j.1365-2567.2012.03584.x. Immunology. 2012. PMID: 22384827 Free PMC article.
References
-
- Brook I. Penicillin failure and copathogenicity in streptococcal pharyngotonsillitis. J Fam Pract. 1994;38:175–179. - PubMed
-
- Paradise JL, et al. Tonsillectomy and adenotonsillectomy for recurrent throat infection in moderately affected children. Pediatrics. 2002;110:7–15. - PubMed
-
- Cunningham MW. Pathogenesis of group A streptococcal infections and their sequelae. Adv Exp Med Biol. 2008;609:29–42. - PubMed
-
- Harrington LE, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

