Cell-cell signal-dependent dynamic interactions between HD-GYP and GGDEF domain proteins mediate virulence in Xanthomonas campestris
- PMID: 20231439
- PMCID: PMC2851925
- DOI: 10.1073/pnas.0912839107
Cell-cell signal-dependent dynamic interactions between HD-GYP and GGDEF domain proteins mediate virulence in Xanthomonas campestris
Erratum in
-
Correction for Ryan et al., Cell-cell signal-dependent dynamic interactions between HD-GYP and GGDEF domain proteins mediate virulence in Xanthomonas campestris.Proc Natl Acad Sci U S A. 2017 Feb 14;114(7):E1303. doi: 10.1073/pnas.1700657114. Epub 2017 Jan 30. Proc Natl Acad Sci U S A. 2017. PMID: 28137834 Free PMC article. No abstract available.
Abstract
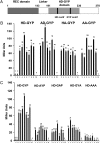
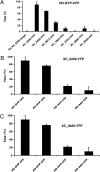
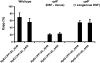
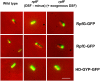
RpfG is a paradigm for a class of widespread bacterial two-component regulators with a CheY-like receiver domain attached to a histidine-aspartic acid-glycine-tyrosine-proline (HD-GYP) cyclic di-GMP phosphodiesterase domain. In the plant pathogen Xanthomonas campestris pv. campestris (Xcc), a two-component system comprising RpfG and the complex sensor kinase RpfC is implicated in sensing and responding to the diffusible signaling factor (DSF), which is essential for cell-cell signaling. RpfF is involved in synthesizing DSF, and mutations of rpfF, rpfG, or rpfC lead to a coordinate reduction in the synthesis of virulence factors such as extracellular enzymes, biofilm structure, and motility. Using yeast two-hybrid analysis and fluorescence resonance energy transfer experiments in Xcc, we show that the physical interaction of RpfG with two proteins with diguanylate cyclase (GGDEF) domains controls a subset of RpfG-regulated virulence functions. RpfG interactions were abolished by alanine substitutions of the three residues of the conserved GYP motif in the HD-GYP domain. Changing the GYP motif or deletion of the two GGDEF-domain proteins reduced Xcc motility but not the synthesis of extracellular enzymes or biofilm formation. RpfG-GGDEF interactions are dynamic and depend on DSF signaling, being reduced in the rpfF mutant but restored by DSF addition. The results are consistent with a model in which DSF signal transduction controlling motility depends on a highly regulated, dynamic interaction of proteins that influence the localized expression of cyclic di-GMP.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Intermolecular interactions between HD-GYP and GGDEF domain proteins mediate virulence-related signal transduction in Xanthomonas campestris.Virulence. 2010 Sep-Oct;1(5):404-8. doi: 10.4161/viru.1.5.12704. Virulence. 2010. PMID: 21178479 Review.
-
Cell-cell signaling in Xanthomonas campestris involves an HD-GYP domain protein that functions in cyclic di-GMP turnover.Proc Natl Acad Sci U S A. 2006 Apr 25;103(17):6712-7. doi: 10.1073/pnas.0600345103. Epub 2006 Apr 12. Proc Natl Acad Sci U S A. 2006. Retraction in: Proc Natl Acad Sci U S A. 2017 Aug 15;114(33):E7031. doi: 10.1073/pnas.1712524114. PMID: 16611728 Free PMC article. Retracted.
-
Dynamic complex formation between HD-GYP, GGDEF and PilZ domain proteins regulates motility in Xanthomonas campestris.Mol Microbiol. 2012 Nov;86(3):557-67. doi: 10.1111/mmi.12000. Epub 2012 Aug 31. Mol Microbiol. 2012. Retraction in: Mol Microbiol. 2017 May;104(3):533. doi: 10.1111/mmi.13670. PMID: 22924852 Retracted.
-
The HD-GYP domain of RpfG mediates a direct linkage between the Rpf quorum-sensing pathway and a subset of diguanylate cyclase proteins in the phytopathogen Xanthomonas axonopodis pv citri.Mol Microbiol. 2006 Oct;62(2):537-51. doi: 10.1111/j.1365-2958.2006.05386.x. Mol Microbiol. 2006. PMID: 17020586
-
The HD-GYP domain, cyclic di-GMP signaling, and bacterial virulence to plants.Mol Plant Microbe Interact. 2006 Dec;19(12):1378-84. doi: 10.1094/MPMI-19-1378. Mol Plant Microbe Interact. 2006. PMID: 17153922 Review.
Cited by
-
Comparative genome analysis unravels pathogenicity of Xanthomonas albilineans causing sugarcane leaf scald disease.BMC Genomics. 2022 Sep 26;23(1):671. doi: 10.1186/s12864-022-08900-2. BMC Genomics. 2022. PMID: 36162999 Free PMC article.
-
Identification of a cyclic-di-GMP-modulating response regulator that impacts biofilm formation in a model sulfate reducing bacterium.Front Microbiol. 2014 Jul 29;5:382. doi: 10.3389/fmicb.2014.00382. eCollection 2014. Front Microbiol. 2014. PMID: 25120537 Free PMC article.
-
Transcriptome-Based Identification of Differently Expressed Genes from Xanthomonas oryzae pv. oryzae Strains Exhibiting Different Virulence in Rice Varieties.Int J Mol Sci. 2016 Feb 19;17(2):259. doi: 10.3390/ijms17020259. Int J Mol Sci. 2016. PMID: 26907259 Free PMC article.
-
The Vibrio cholerae diguanylate cyclase VCA0965 has an AGDEF active site and synthesizes cyclic di-GMP.BMC Microbiol. 2014 Feb 4;14:22. doi: 10.1186/1471-2180-14-22. BMC Microbiol. 2014. PMID: 24490592 Free PMC article.
-
A cyclic GMP-dependent signalling pathway regulates bacterial phytopathogenesis.EMBO J. 2013 Sep 11;32(18):2430-8. doi: 10.1038/emboj.2013.165. Epub 2013 Jul 23. EMBO J. 2013. PMID: 23881098 Free PMC article.
References
-
- Barber CE, et al. A novel regulatory system required for pathogenicity of Xanthomonas campestris is mediated by a small diffusible signal molecule. Mol Microbiol. 1997;24:555–566. - PubMed
-
- Chatterjee S, Sonti RV. rpfF mutants of Xanthomonas oryzae pv. oryzae are deficient for virulence and growth under low iron conditions. Mol Plant Microbe Interact. 2002;15:463–471. - PubMed
-
- Slater H, Alvarez-Morales A, Barber CE, Daniels MJ, Dow JM. A two-component system involving an HD-GYP domain protein links cell-cell signalling to pathogenicity gene expression in Xanthomonas campestris. Mol Microbiol. 2000;38:986–1003. - PubMed
-
- Wang LH, et al. A bacterial cell-cell communication signal with cross-kingdom structural analogues. Mol Microbiol. 2004;51:903–912. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

