Hypothalamic and pituitary c-Jun N-terminal kinase 1 signaling coordinately regulates glucose metabolism
- PMID: 20231445
- PMCID: PMC2851918
- DOI: 10.1073/pnas.1001796107
Hypothalamic and pituitary c-Jun N-terminal kinase 1 signaling coordinately regulates glucose metabolism
Abstract
c-Jun N-terminal kinase (JNK) 1-dependent signaling plays a crucial role in the development of obesity-associated insulin resistance. Here we demonstrate that JNK activation not only occurs in peripheral tissues, but also in the hypothalamus and pituitary of obese mice. To resolve the importance of JNK1 signaling in the hypothalamic/pituitary circuitry, we have generated mice with a conditional inactivation of JNK1 in nestin-expressing cells (JNK1(DeltaNES) mice). JNK1(DeltaNES) mice exhibit improved insulin sensitivity both in the CNS and in peripheral tissues, improved glucose metabolism, as well as protection from hepatic steatosis and adipose tissue dysfunction upon high-fat feeding. Moreover, JNK1(DeltaNES) mice also show reduced somatic growth in the presence of reduced circulating growth hormone (GH) and insulin-like growth factor 1 (IGF1) concentrations, as well as increased thyroid axis activity. Collectively, these experiments reveal an unexpected, critical role for hypothalamic/pituitary JNK1 signaling in the coordination of metabolic/endocrine homeostasis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
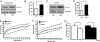

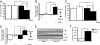
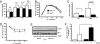

Similar articles
-
Role of the hypothalamic-pituitary-thyroid axis in metabolic regulation by JNK1.Genes Dev. 2010 Feb 1;24(3):256-64. doi: 10.1101/gad.1878510. Epub 2010 Jan 15. Genes Dev. 2010. PMID: 20080940 Free PMC article.
-
Reduction of JNK1 expression with antisense oligonucleotide improves adiposity in obese mice.Am J Physiol Endocrinol Metab. 2008 Aug;295(2):E436-45. doi: 10.1152/ajpendo.00629.2007. Epub 2008 Jun 3. Am J Physiol Endocrinol Metab. 2008. PMID: 18523126
-
Brain JNK and metabolic disease.Diabetologia. 2021 Feb;64(2):265-274. doi: 10.1007/s00125-020-05327-w. Epub 2020 Nov 16. Diabetologia. 2021. PMID: 33200240 Review.
-
Pharmacological characterization of a small molecule inhibitor of c-Jun kinase.Am J Physiol Endocrinol Metab. 2008 Nov;295(5):E1142-51. doi: 10.1152/ajpendo.90298.2008. Epub 2008 Aug 26. Am J Physiol Endocrinol Metab. 2008. PMID: 18728225
-
A transgenic model to determine the physiological role of liver-derived insulin-like growth factor I.Minerva Endocrinol. 2002 Dec;27(4):299-311. Minerva Endocrinol. 2002. PMID: 12511852 Review.
Cited by
-
The essential role of Mbd5 in the regulation of somatic growth and glucose homeostasis in mice.PLoS One. 2012;7(10):e47358. doi: 10.1371/journal.pone.0047358. Epub 2012 Oct 15. PLoS One. 2012. PMID: 23077600 Free PMC article.
-
Hypothalamic inflammation: a double-edged sword to nutritional diseases.Ann N Y Acad Sci. 2011 Dec;1243:E1-39. doi: 10.1111/j.1749-6632.2011.06388.x. Ann N Y Acad Sci. 2011. PMID: 22417140 Free PMC article. Review.
-
JNK signaling contributes to skeletal muscle wasting and protein turnover in pancreatic cancer cachexia.Cancer Lett. 2020 Oct 28;491:70-77. doi: 10.1016/j.canlet.2020.07.025. Epub 2020 Jul 28. Cancer Lett. 2020. PMID: 32735910 Free PMC article.
-
Acute selective ablation of rat insulin promoter-expressing (RIPHER) neurons defines their orexigenic nature.Proc Natl Acad Sci U S A. 2012 Oct 30;109(44):18132-7. doi: 10.1073/pnas.1206147109. Epub 2012 Oct 11. Proc Natl Acad Sci U S A. 2012. PMID: 23064638 Free PMC article.
-
Dicer1-miR-328-Bace1 signalling controls brown adipose tissue differentiation and function.Nat Cell Biol. 2016 Mar;18(3):328-36. doi: 10.1038/ncb3316. Epub 2016 Feb 22. Nat Cell Biol. 2016. PMID: 26900752
References
-
- Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: Direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. - PubMed
-
- Vgontzas AN, et al. Elevation of plasma cytokines in disorders of excessive daytime sleepiness: Role of sleep disturbance and obesity. J Clin Endocrinol Metab. 1997;82:1313–1316. - PubMed
-
- Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. - PubMed
-
- Hirosumi J, et al. A central role for JNK in obesity and insulin resistance. Nature. 2002;420:333–336. - PubMed
-
- Nguyen MT, et al. JNK and tumor necrosis factor-alpha mediate free fatty acid-induced insulin resistance in 3T3-L1 adipocytes. J Biol Chem. 2005;280:35361–35371. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

