Huwe1 ubiquitin ligase is essential to synchronize neuronal and glial differentiation in the developing cerebellum
- PMID: 20231446
- PMCID: PMC2851916
- DOI: 10.1073/pnas.0912874107
Huwe1 ubiquitin ligase is essential to synchronize neuronal and glial differentiation in the developing cerebellum
Abstract
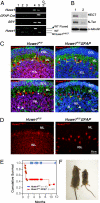
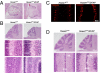
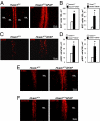
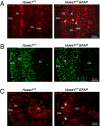
We have generated a knockout mouse strain in which the gene coding for the ubiquitin ligase Huwe1 has been inactivated in cerebellar granule neuron precursors (CGNPs) and radial glia. These mice have a high rate of postnatal lethality and profound cerebellar abnormalities. The external granule layer of the cerebellum, which contains CGNPs, is expanded and displays aberrant proliferation and impaired differentiation of the progenitor cell population. The uncontrolled proliferation of the CGNPs is associated with accumulation of the N-Myc oncoprotein, a substrate of Huwe1, and con-sequent activation of the signaling events downstream to N-Myc. Furthermore, loss of Huwe1 in Bergmann glia leads to extensive disorganization of this cell population with layering aberrations, severe granule neuron migration defects, and persistence of ectopic clusters of granule neurons in the external granule layer. Our findings uncover an unexpected role for Huwe1 in regulating Berg-mann glia differentiation and indicate that this ubiquitin ligase orchestrates the programming of the neural progenitors that give rise to neurons and glia in the cerebellum.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Glial fibrillary acidic protein-expressing neural progenitors give rise to immature neurons via early intermediate progenitors expressing both glial fibrillary acidic protein and neuronal markers in the adult hippocampus.Neuroscience. 2010 Mar 10;166(1):241-51. doi: 10.1016/j.neuroscience.2009.12.026. Epub 2009 Dec 16. Neuroscience. 2010. PMID: 20026190
-
The N-Myc-DLL3 cascade is suppressed by the ubiquitin ligase Huwe1 to inhibit proliferation and promote neurogenesis in the developing brain.Dev Cell. 2009 Aug;17(2):210-21. doi: 10.1016/j.devcel.2009.07.009. Dev Cell. 2009. PMID: 19686682 Free PMC article.
-
Transcriptional Regulator ZEB2 Is Essential for Bergmann Glia Development.J Neurosci. 2018 Feb 7;38(6):1575-1587. doi: 10.1523/JNEUROSCI.2674-17.2018. Epub 2018 Jan 11. J Neurosci. 2018. PMID: 29326173 Free PMC article.
-
Bergmann glia function in granule cell migration during cerebellum development.Mol Neurobiol. 2013 Apr;47(2):833-44. doi: 10.1007/s12035-013-8405-y. Epub 2013 Jan 19. Mol Neurobiol. 2013. PMID: 23329344 Review.
-
Cytodifferentiation of Bergmann glia and its relationship with Purkinje cells.Anat Sci Int. 2002 Jun;77(2):94-108. doi: 10.1046/j.0022-7722.2002.00021.x. Anat Sci Int. 2002. PMID: 12418089 Review.
Cited by
-
Novel Genetic Variant in HUWE1: Prenatal and Postnatal Neuroimaging Phenotype.Neurol Genet. 2024 Jun 13;10(4):e200169. doi: 10.1212/NXG.0000000000200169. eCollection 2024 Aug. Neurol Genet. 2024. PMID: 39139262 Free PMC article.
-
Ubiquitin Ligase Huwe1 Modulates Spermatogenesis by Regulating Spermatogonial Differentiation and Entry into Meiosis.Sci Rep. 2017 Dec 19;7(1):17759. doi: 10.1038/s41598-017-17902-0. Sci Rep. 2017. PMID: 29259204 Free PMC article.
-
Cerebellar Astrocytes: Much More Than Passive Bystanders In Ataxia Pathophysiology.J Clin Med. 2020 Mar 11;9(3):757. doi: 10.3390/jcm9030757. J Clin Med. 2020. PMID: 32168822 Free PMC article. Review.
-
Mule/Huwe1/Arf-BP1 suppresses Ras-driven tumorigenesis by preventing c-Myc/Miz1-mediated down-regulation of p21 and p15.Genes Dev. 2013 May 15;27(10):1101-14. doi: 10.1101/gad.214577.113. Genes Dev. 2013. PMID: 23699408 Free PMC article.
-
New insights into craniofacial malformations.Hum Mol Genet. 2015 Oct 15;24(R1):R50-9. doi: 10.1093/hmg/ddv228. Epub 2015 Jun 17. Hum Mol Genet. 2015. PMID: 26085576 Free PMC article. Review.
References
-
- Chanas-Sacre G, Rogister B, Moonen G, Leprince P. Radial glia phenotype: Origin, regulation, and transdifferentiation. J Neurosci Res. 2000;61:357–363. - PubMed
-
- Yamada K, Watanabe M. Cytodifferentiation of Bergmann glia and its relationship with Purkinje cells. Anat Sci Int. 2002;77:94–108. - PubMed
-
- Zhuo L, et al. hGFAP-cre transgenic mice for manipulation of glial and neuronal function in vivo. Genesis. 2001;31:85–94. - PubMed
-
- Yamada K, et al. Dynamic transformation of Bergmann glial fibers proceeds in correlation with dendritic outgrowth and synapse formation of cerebellar Purkinje cells. J Comp Neurol. 2000;418:106–120. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

