Identification of hepatoprotective flavonolignans from silymarin
- PMID: 20231449
- PMCID: PMC2851903
- DOI: 10.1073/pnas.0914009107
Identification of hepatoprotective flavonolignans from silymarin
Abstract
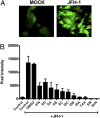
Silymarin, also known as milk thistle extract, inhibits hepatitis C virus (HCV) infection and also displays antioxidant, anti-inflammatory, and immunomodulatory actions that contribute to its hepatoprotective effects. In the current study, we evaluated the hepatoprotective actions of the seven major flavonolignans and one flavonoid that comprise silymarin. Activities tested included inhibition of: HCV cell culture infection, NS5B polymerase activity, TNF-alpha-induced NF-kappaB transcription, virus-induced oxidative stress, and T-cell proliferation. All compounds were well tolerated by Huh7 human hepatoma cells up to 80 muM, except for isosilybin B, which was toxic to cells above 10 muM. Select compounds had stronger hepatoprotective functions than silymarin in all assays tested except in T cell proliferation. Pure compounds inhibited JFH-1 NS5B polymerase but only at concentrations above 300 muM. Silymarin suppressed TNF-alpha activation of NF-kappaB dependent transcription, which involved partial inhibition of IkappaB and RelA/p65 serine phosphorylation, and p50 and p65 nuclear translocation, without affecting binding of p50 and p65 to DNA. All compounds blocked JFH-1 virus-induced oxidative stress, including compounds that lacked antiviral activity. The most potent compounds across multiple assays were taxifolin, isosilybin A, silybin A, silybin B, and silibinin, a mixture of silybin A and silybin B. The data suggest that silymarin- and silymarin-derived compounds may influence HCV disease course in some patients. Studies where standardized silymarin is dosed to identify specific clinical endpoints are urgently needed.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

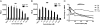
Similar articles
-
Inhibition of T-cell inflammatory cytokines, hepatocyte NF-kappaB signaling, and HCV infection by standardized Silymarin.Gastroenterology. 2007 May;132(5):1925-36. doi: 10.1053/j.gastro.2007.02.038. Epub 2007 Feb 21. Gastroenterology. 2007. PMID: 17484885
-
Milk thistle and prostate cancer: differential effects of pure flavonolignans from Silybum marianum on antiproliferative end points in human prostate carcinoma cells.Cancer Res. 2005 May 15;65(10):4448-57. doi: 10.1158/0008-5472.CAN-04-4662. Cancer Res. 2005. PMID: 15899838
-
Influence of silymarin and its flavonolignans on H(2)O(2)-induced oxidative stress in human keratinocytes and mouse fibroblasts.Burns. 2006 Dec;32(8):973-9. doi: 10.1016/j.burns.2006.04.004. Epub 2006 Sep 29. Burns. 2006. PMID: 17011711
-
Antiviral Activities of Silymarin and Derivatives.Molecules. 2019 Apr 19;24(8):1552. doi: 10.3390/molecules24081552. Molecules. 2019. PMID: 31010179 Free PMC article. Review.
-
"Non-Taxifolin" Derived Flavonolignans: Phytochemistry and Biology.Curr Pharm Des. 2015;21(38):5489-500. doi: 10.2174/1381612821666151002112720. Curr Pharm Des. 2015. PMID: 26429716 Review.
Cited by
-
Hepatitis C virus and natural compounds: a new antiviral approach?Viruses. 2012 Oct 17;4(10):2197-217. doi: 10.3390/v4102197. Viruses. 2012. PMID: 23202460 Free PMC article. Review.
-
Enhanced bioactivity of silybin B methylation products.Bioorg Med Chem. 2013 Feb 1;21(3):742-7. doi: 10.1016/j.bmc.2012.11.035. Epub 2012 Dec 5. Bioorg Med Chem. 2013. PMID: 23260576 Free PMC article.
-
Chirality Matters: Biological Activity of Optically Pure Silybin and Its Congeners.Int J Mol Sci. 2021 Jul 23;22(15):7885. doi: 10.3390/ijms22157885. Int J Mol Sci. 2021. PMID: 34360650 Free PMC article. Review.
-
Botanicals and Their Bioactive Phytochemicals for Women's Health.Pharmacol Rev. 2016 Oct;68(4):1026-1073. doi: 10.1124/pr.115.010843. Pharmacol Rev. 2016. PMID: 27677719 Free PMC article. Review.
-
Carbon Monoxide-Releasing Activity of Plant Flavonoids.J Agric Food Chem. 2025 Jan 15;73(2):1308-1318. doi: 10.1021/acs.jafc.4c09069. Epub 2024 Dec 31. J Agric Food Chem. 2025. PMID: 39740217 Free PMC article.
References
-
- Davis GL, Albright JE, Cook SF, Rosenberg DM. Projecting future complications of chronic hepatitis C in the United States. Liver Transpl. 2003;9:331–338. - PubMed
-
- Alter HJ. HCV natural history: the retrospective and prospective in perspective. J Hepatol. 2005;43:550–552. - PubMed
-
- Manns MP, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958–965. - PubMed
-
- Fried MW, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975–982. - PubMed
-
- Nelson DR. Hepatitis C drug development at a crossroads. Hepatology. 2009;50:997–999. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

