Polymorphonuclear leukocytes mediate Staphylococcus aureus Panton-Valentine leukocidin-induced lung inflammation and injury
- PMID: 20231457
- PMCID: PMC2851770
- DOI: 10.1073/pnas.0912403107
Polymorphonuclear leukocytes mediate Staphylococcus aureus Panton-Valentine leukocidin-induced lung inflammation and injury
Abstract
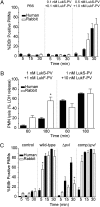
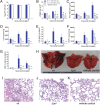
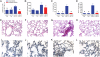
Community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA) is epidemic in the United States, even rivaling HIV/AIDS in its public health impact. The pandemic clone USA300, like other CA-MRSA strains, expresses Panton-Valentine leukocidin (PVL), a pore-forming toxin that targets polymorphonuclear leukocytes (PMNs). PVL is thought to play a key role in the pathogenesis of necrotizing pneumonia, but data from rodent infection models are inconclusive. Rodent PMNs are less susceptible than human PMNs to PVL-induced cytolysis, whereas rabbit PMNs, like those of humans, are highly susceptible to PVL-induced cytolysis. This difference in target cell susceptibility could affect results of experimental models. Therefore, we developed a rabbit model of necrotizing pneumonia to compare the virulence of a USA300 wild-type strain with that of isogenic PVL-deletion mutant and -complemented strains. PVL enhanced the capacity of USA300 to cause severe lung necrosis, pulmonary edema, alveolar hemorrhage, hemoptysis, and death, hallmark clinical features of fatal human necrotizing pneumonia. Purified PVL instilled directly into the lung caused lung inflammation and injury by recruiting and lysing PMNs, which damage the lung by releasing cytotoxic granule contents. These findings provide insights into the mechanism of PVL-induced lung injury and inflammation and demonstrate the utility of the rabbit for studying PVL-mediated pathogenesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Klevens RM, et al. Active Bacterial Core surveillance (ABCs) MRSA Investigators. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA. 2007;298:1763–1771. - PubMed
-
- Gillet Y, et al. Association between Staphylococcus aureus strains carrying gene for Panton-Valentine leukocidin and highly lethal necrotising pneumonia in young immunocompetent patients. Lancet. 2002;359:753–759. - PubMed
-
- Hidron AI, Low CE, Honig EG, Blumberg HM. Emergence of community-acquired meticillin-resistant Staphylococcus aureus strain USA300 as a cause of necrotising community-onset pneumonia. Lancet Infect Dis. 2009;9:384–392. - PubMed
-
- Francis JS, et al. Severe community-onset pneumonia in healthy adults caused by methicillin-resistant Staphylococcus aureus carrying the Panton-Valentine leukocidin genes. Clin Infect Dis. 2005;40:100–107. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

