Mechanisms of acute axonal degeneration in the optic nerve in vivo
- PMID: 20231460
- PMCID: PMC2851885
- DOI: 10.1073/pnas.0909794107
Mechanisms of acute axonal degeneration in the optic nerve in vivo
Abstract
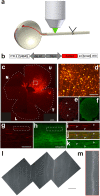
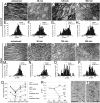
Axonal degeneration is an initial key step in traumatic and neurodegenerative CNS disorders. We established a unique in vivo epifluorescence imaging paradigm to characterize very early events in axonal degeneration in the rat optic nerve. Single retinal ganglion cell axons were visualized by AAV-mediated expression of dsRed and this allowed the quantification of postlesional acute axonal degeneration (AAD). EM analysis revealed severe structural alterations of the cytoskeleton, cytoplasmatic vacuolization, and the appearance of autophagosomes within the first hours after lesion. Inhibition of autophagy resulted in an attenuation of acute axonal degeneration. Furthermore, a rapid increase of intraaxonal calcium levels following crush lesion could be visualized using a calcium-sensitive dye. Application of calcium channel inhibitors prevented crush-induced calcium increase and markedly attenuated axonal degeneration, whereas application of a calcium ionophore aggravated the degenerative phenotype. We finally demonstrate that increased postlesional autophagy is calcium dependent and thus mechanistically link autophagy and intraaxonal calcium levels. Both processes are proposed to be major targets for the manipulation of axonal degeneration in future therapeutic settings.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

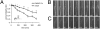


References
-
- Hagg T, Oudega M. Degenerative and spontaneous regenerative processes after spinal cord injury. J Neurotrauma. 2006;23:264–280. - PubMed
-
- Navarro X. Chapter 27: Neural plasticity after nerve injury and regeneration. Int Rev Neurobiol. 2009;87:483–505. - PubMed
-
- Orimo S, et al. Axonal alpha-synuclein aggregates herald centripetal degeneration of cardiac sympathetic nerve in Parkinson's disease. Brain. 2008;131:642–650. - PubMed
-
- Fischer LR, et al. Amyotrophic lateral sclerosis is a distal axonopathy: Evidence in mice and man. Exp Neurol. 2004;185:232–240. - PubMed
-
- Cavanagh JB. The significance of the “dying back” process in experimental and human neurological disease. Int Rev Exp Pathol. 1964;3:219–267. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

