Dendritic cells control T cell tonic signaling required for responsiveness to foreign antigen
- PMID: 20231464
- PMCID: PMC2851879
- DOI: 10.1073/pnas.0911877107
Dendritic cells control T cell tonic signaling required for responsiveness to foreign antigen
Abstract
Dendritic cells (DCs) are key components of the adaptive immune system contributing to initiation and regulation of T cell responses. T cells continuously scan DCs in lymphoid organs for the presence of foreign antigen. However, little is known about the functional consequences of these frequent T cell-DC interactions without cognate antigen. Here we demonstrate that these contacts in the absence of foreign antigen serve an important function, namely, induction of a basal activation level in T cells required for responsiveness to subsequent encounters with foreign antigens. This basal activation is provided by self-recognition of MHC molecules on DCs. Following DC depletion in mice, T cells became impaired in TCR signaling and immune synapse formation, and consequently were hyporesponsive to antigen. This process was reversible, as T cells quickly recovered when the number of DCs returned to a normal level. The extent of T cell reactivity correlated with the degree of DC depletion in lymphoid organs, suggesting that a full DC compartment guarantees optimal T cell responsiveness. These findings indicate that DCs are specialized cells that not only present foreign antigen, but also promote a "tonic" state in T cells for antigen responsiveness.
Conflict of interest statement
The authors declare no conflict of interest.
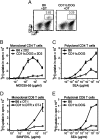
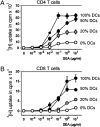
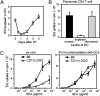
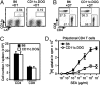
Figures


References
-
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. - PubMed
-
- Steinman RM. Lasker Basic Medical Research Award. Dendritic cells: Versatile controllers of the immune system. Nat Med. 2007;13:1155–1159. - PubMed
-
- Shortman K, Liu YJ. Mouse and human dendritic cell subtypes. Nat Rev Immunol. 2002;2:151–161. - PubMed
-
- Villadangos JA, Schnorrer P. Intrinsic and cooperative antigen-presenting functions of dendritic-cell subsets in vivo. Nat Rev Immunol. 2007;7:543–555. - PubMed
-
- Jenkins MK, et al. In vivo activation of antigen-specific CD4 T cells. Annu Rev Immunol. 2001;19:23–45. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

