Diabetes-accelerated memory dysfunction via cerebrovascular inflammation and Abeta deposition in an Alzheimer mouse model with diabetes
- PMID: 20231468
- PMCID: PMC2872449
- DOI: 10.1073/pnas.1000645107
Diabetes-accelerated memory dysfunction via cerebrovascular inflammation and Abeta deposition in an Alzheimer mouse model with diabetes
Abstract
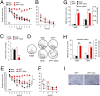
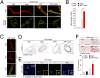
Recent epidemiological studies suggest that diabetes mellitus is a strong risk factor for Alzheimer disease. However, the underlying mechanisms remain largely unknown. In this study, to investigate the pathophysiological interaction between these diseases, we generated animal models that reflect the pathologic conditions of both diseases. We crossed Alzheimer transgenic mice (APP23) with two types of diabetic mice (ob/ob and NSY mice), and analyzed their metabolic and brain pathology. The onset of diabetes exacerbated Alzheimer-like cognitive dysfunction without an increase in brain amyloid-beta burden in double-mutant (APP(+)-ob/ob) mice. Notably, APP(+)-ob/ob mice showed cerebrovascular inflammation and severe amyloid angiopathy. Conversely, the cross-bred mice showed an accelerated diabetic phenotype compared with ob/ob mice, suggesting that Alzheimer amyloid pathology could aggravate diabetes. Similarly, APP(+)-NSY fusion mice showed more severe glucose intolerance compared with diabetic NSY mice. Furthermore, high-fat diet feeding induced severe memory deficits in APP(+)-NSY mice without an increase in brain amyloid-beta load. Here, we created Alzheimer mouse models with early onset of cognitive dysfunction. Cerebrovascular changes and alteration in brain insulin signaling might play a pivotal role in this relationship. These findings could provide insights into this intensely debated association.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Comment in
-
Linking type 2 diabetes and Alzheimer's disease.Proc Natl Acad Sci U S A. 2010 Apr 13;107(15):6557-8. doi: 10.1073/pnas.1002555107. Epub 2010 Apr 12. Proc Natl Acad Sci U S A. 2010. PMID: 20385830 Free PMC article. No abstract available.
References
-
- Flier JS. Obesity wars: molecular progress confronts an expanding epidemic. Cell. 2004;116:337–350. - PubMed
-
- Maher PA, Schubert DR. Metabolic links between diabetes and Alzheimer's disease. Expert Rev Neurother. 2009;9:617–630. - PubMed
-
- Ott A, et al. Diabetes mellitus and the risk of dementia: The Rotterdam Study. Neurology. 1999;53:1937–1942. - PubMed
-
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. - PubMed
-
- Gasparini L, Netzer WJ, Greengard P, Xu H. Does insulin dysfunction play a role in Alzheimer's disease? Trends Pharmacol Sci. 2002;23:288–293. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

