Brassinosteroids control male fertility by regulating the expression of key genes involved in Arabidopsis anther and pollen development
- PMID: 20231470
- PMCID: PMC2851861
- DOI: 10.1073/pnas.0912333107
Brassinosteroids control male fertility by regulating the expression of key genes involved in Arabidopsis anther and pollen development
Abstract
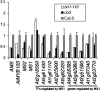
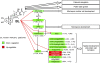
The development of anther and pollen is important for male reproduction, and this process is coordinately regulated by many external and internal cues. In this study, we systematically examined the male reproductive phenotypes of a series of brassinosteroid biosynthetic and signaling mutants and found that, besides the expected cell-expansion defects, these mutants also showed reduced pollen number, viability, and release efficiency. These defects were related with abnormal tapetum and microspore development. Using both real-time quantitative RT-PCR and microarray experiments, we found that the expression of many key genes required for anther and pollen development was suppressed in these mutants. ChIP analysis demonstrated that BES1, an important transcription factor for brassinosteroid signaling, could directly bind to the promoter regions of genes encoding transcription factors essential for anther and pollen development, SPL/NZZ, TDF1, AMS, MS1, and MS2. Taken together, these data lead us to propose that brassinosteroids control male fertility at least in part via directly regulating key genes for anther and pollen development in Arabidopsis. Our work provides a unique mechanism to explain how a phytohormone regulates an essential genetic program for plant development.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
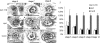

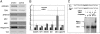
Similar articles
-
Regulation of the Arabidopsis anther transcriptome by DYT1 for pollen development.Plant J. 2012 Nov;72(4):612-24. doi: 10.1111/j.1365-313X.2012.05104.x. Epub 2012 Sep 20. Plant J. 2012. PMID: 22775442
-
Epidermal jasmonate perception is sufficient for all aspects of jasmonate-mediated male fertility in Arabidopsis.Plant J. 2016 Mar;85(5):634-47. doi: 10.1111/tpj.13131. Plant J. 2016. PMID: 26833563
-
ECHIDNA protein impacts on male fertility in Arabidopsis by mediating trans-Golgi network secretory trafficking during anther and pollen development.Plant Physiol. 2014 Mar;164(3):1338-49. doi: 10.1104/pp.113.227769. Epub 2014 Jan 14. Plant Physiol. 2014. PMID: 24424320 Free PMC article.
-
The final split: the regulation of anther dehiscence.J Exp Bot. 2011 Mar;62(5):1633-49. doi: 10.1093/jxb/err014. Epub 2011 Feb 16. J Exp Bot. 2011. PMID: 21325605 Review.
-
Transcriptional regulation of anther development in Arabidopsis.Gene. 2019 Mar 20;689:202-209. doi: 10.1016/j.gene.2018.12.022. Epub 2018 Dec 17. Gene. 2019. PMID: 30572098 Review.
Cited by
-
Local brassinosteroid biosynthesis enables optimal root growth.Nat Plants. 2021 May;7(5):619-632. doi: 10.1038/s41477-021-00917-x. Epub 2021 May 17. Nat Plants. 2021. PMID: 34007032
-
Regulation of the nuclear activities of brassinosteroid signaling.Curr Opin Plant Biol. 2010 Oct;13(5):540-7. doi: 10.1016/j.pbi.2010.08.007. Epub 2010 Sep 17. Curr Opin Plant Biol. 2010. PMID: 20851039 Free PMC article. Review.
-
Brassinosteroids: Multidimensional Regulators of Plant Growth, Development, and Stress Responses.Plant Cell. 2020 Feb;32(2):295-318. doi: 10.1105/tpc.19.00335. Epub 2019 Nov 27. Plant Cell. 2020. PMID: 31776234 Free PMC article. Review.
-
PcDWF1, a pear brassinosteroid biosynthetic gene homologous to AtDWARF1, affected the vegetative and reproductive growth of plants.BMC Plant Biol. 2020 Mar 6;20(1):109. doi: 10.1186/s12870-020-2323-8. BMC Plant Biol. 2020. PMID: 32143576 Free PMC article.
-
PbrBZR1 interacts with PbrARI2.3 to mediate brassinosteroid-regulated pollen tube growth during self-incompatibility signaling in pear.Plant Physiol. 2023 Jul 3;192(3):2356-2373. doi: 10.1093/plphys/kiad208. Plant Physiol. 2023. PMID: 37010117 Free PMC article.
References
-
- Allison AS, Paul OL. Reproductive traits and male fertility in plants: empirical approaches. Annu Rev Ecol Syst. 1993;24:331–351.
-
- Ma H. Molecular genetic analyses of microsporogenesis and microgametogenesis in flowering plants. Annu Rev Plant Biol. 2005;56:393–434. - PubMed
-
- Wijeratne AJ, et al. Differential gene expression in Arabidopsis wild-type and mutant anther: insights into anther cell differentiation and regulatory networks. Plant J. 2007;52:14–29. - PubMed
-
- Balasubramanian S, Schneitz K. NOZZLE regulates proximal-distal pattern formation, cell proliferation and early sporogenesis during ovule development in Arabidopsis thaliana. Development. 2000;127:4227–4238. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

