The neural code of reward anticipation in human orbitofrontal cortex
- PMID: 20231475
- PMCID: PMC2851854
- DOI: 10.1073/pnas.0912838107
The neural code of reward anticipation in human orbitofrontal cortex
Abstract
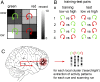
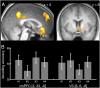
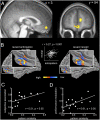
An optimal choice among alternative behavioral options requires precise anticipatory representations of their possible outcomes. A fundamental question is how such anticipated outcomes are represented in the brain. Reward coding at the level of single cells in the orbitofrontal cortex (OFC) follows a more heterogeneous coding scheme than suggested by studies using functional MRI (fMRI) in humans. Using a combination of multivariate pattern classification and fMRI we show that the reward value of sensory cues can be decoded from distributed fMRI patterns in the OFC. This distributed representation is compatible with previous reports from animal electrophysiology that show that reward is encoded by different neural populations with opposing coding schemes. Importantly, the fMRI patterns representing specific values during anticipation are similar to those that emerge during the receipt of reward. Furthermore, we show that the degree of this coding similarity is related to subjects' ability to use value information to guide behavior. These findings narrow the gap between reward coding in humans and animals and corroborate the notion that value representations in OFC are independent of whether reward is anticipated or actually received.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Outcome representations, counterfactual comparisons and the human orbitofrontal cortex: implications for neuroimaging studies of decision-making.Brain Res Cogn Brain Res. 2005 Apr;23(1):51-60. doi: 10.1016/j.cogbrainres.2005.01.004. Brain Res Cogn Brain Res. 2005. PMID: 15795133 Clinical Trial.
-
Identity-Specific Reward Representations in Orbitofrontal Cortex Are Modulated by Selective Devaluation.J Neurosci. 2017 Mar 8;37(10):2627-2638. doi: 10.1523/JNEUROSCI.3473-16.2017. Epub 2017 Feb 3. J Neurosci. 2017. PMID: 28159906 Free PMC article.
-
Reward-related reversal learning after surgical excisions in orbito-frontal or dorsolateral prefrontal cortex in humans.J Cogn Neurosci. 2004 Apr;16(3):463-78. doi: 10.1162/089892904322926791. J Cogn Neurosci. 2004. PMID: 15072681
-
Neural representations of subjective reward value.Behav Brain Res. 2010 Dec 1;213(2):135-41. doi: 10.1016/j.bbr.2010.04.031. Epub 2010 Apr 24. Behav Brain Res. 2010. PMID: 20420859 Review.
-
Contrasting reward signals in the orbitofrontal cortex and anterior cingulate cortex.Ann N Y Acad Sci. 2011 Dec;1239:33-42. doi: 10.1111/j.1749-6632.2011.06277.x. Ann N Y Acad Sci. 2011. PMID: 22145873 Review.
Cited by
-
The neural basis of temporal individuation and its capacity limits in the human brain.J Neurophysiol. 2014 Feb;111(3):499-512. doi: 10.1152/jn.00534.2013. Epub 2013 Nov 6. J Neurophysiol. 2014. Retraction in: J Neurophysiol. 2016 Nov 1;116(5):2467. doi: 10.1152/jn.z9k-3963-retr.2016. PMID: 24198320 Free PMC article. Retracted.
-
BOLD subjective value signals exhibit robust range adaptation.J Neurosci. 2014 Dec 3;34(49):16533-43. doi: 10.1523/JNEUROSCI.3927-14.2014. J Neurosci. 2014. PMID: 25471589 Free PMC article.
-
The neural basis of temporal individuation and its capacity limits in the human brain.J Neurophysiol. 2017 Nov 1;118(5):2601-2613. doi: 10.1152/jn.00839.2016. Epub 2017 Aug 30. J Neurophysiol. 2017. PMID: 28855297 Free PMC article.
-
Effect of carbonated water on cerebral blood flow in the frontal region: a study using near-infrared spectroscopy.Front Behav Neurosci. 2024 Dec 10;18:1409123. doi: 10.3389/fnbeh.2024.1409123. eCollection 2024. Front Behav Neurosci. 2024. PMID: 39720307 Free PMC article.
-
Differential maturation of the brain networks required for the sensory, emotional, and cognitive aspects of pain in human newborns.Pain. 2025 Jun 18:10.1097/j.pain.0000000000003619. doi: 10.1097/j.pain.0000000000003619. Online ahead of print. Pain. 2025. PMID: 40532739 Free PMC article.
References
-
- Rolls ET. The orbitofrontal cortex and reward. Cereb Cortex. 2000;10:284–294. - PubMed
-
- Kringelbach ML. The human orbitofrontal cortex: Linking reward to hedonic experience. Nat Rev Neurosci. 2005;6:691–702. - PubMed
-
- O'Doherty JP. Lights, camembert, action! The role of human orbitofrontal cortex in encoding stimuli, rewards and choices. Ann N Y Acad Sci. 2007;1121:254–272. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

