Locus ceruleus controls Alzheimer's disease pathology by modulating microglial functions through norepinephrine
- PMID: 20231476
- PMCID: PMC2851853
- DOI: 10.1073/pnas.0909586107
Locus ceruleus controls Alzheimer's disease pathology by modulating microglial functions through norepinephrine
Abstract
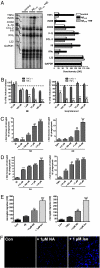
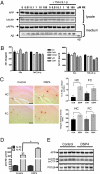
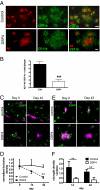
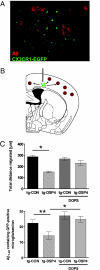
Locus ceruleus (LC)-supplied norepinephrine (NE) suppresses neuroinflammation in the brain. To elucidate the effect of LC degeneration and subsequent NE deficiency on Alzheimer's disease pathology, we evaluated NE effects on microglial key functions. NE stimulation of mouse microglia suppressed Abeta-induced cytokine and chemokine production and increased microglial migration and phagocytosis of Abeta. Induced degeneration of the locus ceruleus increased expression of inflammatory mediators in APP-transgenic mice and resulted in elevated Abeta deposition. In vivo laser microscopy confirmed a reduced recruitment of microglia to Abeta plaque sites and impaired microglial Abeta phagocytosis in NE-depleted APP-transgenic mice. Supplying the mice the norepinephrine precursor L-threo-DOPS restored microglial functions in NE-depleted mice. This indicates that decrease of NE in locus ceruleus projection areas facilitates the inflammatory reaction of microglial cells in AD and impairs microglial migration and phagocytosis, thereby contributing to reduced Abeta clearance. Consequently, therapies targeting microglial phagocytosis should be tested under NE depletion.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Comment in
-
Neurodegenerative diseases: microglia's little helper.Nat Rev Neurosci. 2010 May;11(5):297. doi: 10.1038/nrn2843. Nat Rev Neurosci. 2010. PMID: 20419860 No abstract available.
References
-
- Forno L. Pathology of Parkinsonism: A preliminary report of 24 cases. J Neurosurg. 1966;(Supplement, Part II):266–271.
-
- Iversen LL, et al. Loss of pigmented dopamine-beta-hydroxylase positive cells from locus coeruleus in senile dementia of Alzheimer's type. Neurosci Lett. 1983;39:95–100. - PubMed
-
- Bondareff W, et al. Neuronal degeneration in locus ceruleus and cortical correlates of Alzheimer disease. Alzheimer Dis Assoc Disord. 1987;1:256–262. - PubMed
-
- Matthews KL, et al. Noradrenergic changes, aggressive behavior, and cognition in patients with dementia. Biol Psychiatry. 2002;51:407–416. - PubMed
-
- Grudzien A, et al. Locus coeruleus neurofibrillary degeneration in aging, mild cognitive impairment and early Alzheimer's disease. Neurobiol Aging. 2007;28:327–335. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

