L-amino acids catalyze the formation of an excess of D-glyceraldehyde, and thus of other D sugars, under credible prebiotic conditions
- PMID: 20231487
- PMCID: PMC2851924
- DOI: 10.1073/pnas.1001639107
L-amino acids catalyze the formation of an excess of D-glyceraldehyde, and thus of other D sugars, under credible prebiotic conditions
Abstract
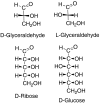
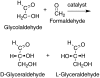
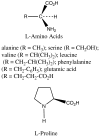
Previous work by us, and others, has shown that the formation of amino acids on prebiotic earth with the geometric arrangement called the L configuration can be understood. Some meteorites of the carbonaceous chondritic type deliver unusual amino acids, with alpha-methyl groups, which have an excess of the L isomers. We previously showed that in decarboxylative transamination reactions under credible prebiotic conditions they produce normal amino acids that also have a preference for the L isomer, as is found in our proteins. We, and others, showed that as little as a 1% excess of the L isomers could be amplified up to a 95/5 ratio of L over D on simple evaporation of a solution, so life could start with such a solution in which the dominant L isomers would be selectively chosen. We now find that the geometry of sugars referred to D, as in D-ribose or D-glucose, is not an independent mystery. D-glyceraldehyde, the simplest sugar with a D center, is the basic unit on which other sugars are built. We find that the synthesis of glyceraldehyde by reaction of formaldehyde with glycolaldehyde is catalyzed under prebiotic conditions to D/L ratios greater than 1, to as much as 60/40, by a representative group of L-amino acids (with the exception of L-proline). The D/L glyceraldehyde ratio in water solution is amplified to 92/8 using simple selective solubilities of the D and the DL forms. This D center would then be carried into the prebiotic syntheses of larger sugars.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Catalysis of glyceraldehyde synthesis by primary or secondary amino acids under prebiotic conditions as a function of pH.Orig Life Evol Biosph. 2013 Oct;43(4-5):323-9. doi: 10.1007/s11084-013-9347-0. Epub 2013 Dec 18. Orig Life Evol Biosph. 2013. PMID: 24346788
-
On the origin of single chirality of amino acids and sugars in biogenesis.Acc Chem Res. 2012 Dec 18;45(12):2045-54. doi: 10.1021/ar200316n. Epub 2012 Feb 22. Acc Chem Res. 2012. PMID: 22353168
-
Imitating prebiotic homochirality on Earth.Orig Life Evol Biosph. 2010 Feb;40(1):11-26. doi: 10.1007/s11084-009-9179-0. Epub 2009 Nov 13. Orig Life Evol Biosph. 2010. PMID: 19911303
-
Current status of the prebiotic synthesis of small molecules.Chem Scr. 1986;26B:5-11. Chem Scr. 1986. PMID: 11542054 Review.
-
Understanding prebiotic chemistry through the analysis of extraterrestrial amino acids and nucleobases in meteorites.Chem Soc Rev. 2012 Aug 21;41(16):5459-72. doi: 10.1039/c2cs35109a. Epub 2012 Jun 15. Chem Soc Rev. 2012. PMID: 22706603 Review.
Cited by
-
The progene hypothesis: the nucleoprotein world and how life began.Biol Direct. 2015 Nov 26;10:67. doi: 10.1186/s13062-015-0096-z. Biol Direct. 2015. PMID: 26612610 Free PMC article. Review.
-
Multicomponent assembly of proposed DNA precursors in water.J Am Chem Soc. 2012 Aug 22;134(33):13889-95. doi: 10.1021/ja306176n. Epub 2012 Aug 9. J Am Chem Soc. 2012. PMID: 22839703 Free PMC article.
-
Catalysis of glyceraldehyde synthesis by primary or secondary amino acids under prebiotic conditions as a function of pH.Orig Life Evol Biosph. 2013 Oct;43(4-5):323-9. doi: 10.1007/s11084-013-9347-0. Epub 2013 Dec 18. Orig Life Evol Biosph. 2013. PMID: 24346788
-
Formose Reaction Controlled by a Copolymer of N,N-Dimethylacrylamide and 4-Vinylphenylboronic Acid.Polymers (Basel). 2017 Oct 25;9(11):549. doi: 10.3390/polym9110549. Polymers (Basel). 2017. PMID: 30965856 Free PMC article.
-
On the Origin of Sugar Handedness: Facts, Hypotheses and Missing Links-A Review.Orig Life Evol Biosph. 2022 Sep;52(1-3):21-56. doi: 10.1007/s11084-022-09624-9. Epub 2022 Jul 7. Orig Life Evol Biosph. 2022. PMID: 35796896 Review.
References
-
- Cronin J, Pizzarello S. Enantiomeric excesses in meteoritic amino acids. Science. 1997;275:951–955. - PubMed
-
- Pizzarello S. The chemistry of life’s origin: A carbonaceous meteorite perspective. Acc Chem Res. 2006;39:231–235. - PubMed
-
- Levine M, Kenesky C, Mazori D, Breslow R. Enantioselective synthesis and enantiomeric amplification of amino acids under prebiotic conditions. Org Lett. 2008;10:2433–2436. - PubMed
-
- Klussman M, et al. Thermodynamic control of asymmetric amplification in amino acid catalysis. Nature. 2006;441:621–623. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

