Protein-DNA complex is the exclusive malaria parasite component that activates dendritic cells and triggers innate immune responses
- PMID: 20231693
- PMCID: PMC2851449
- DOI: 10.4049/jimmunol.0903824
Protein-DNA complex is the exclusive malaria parasite component that activates dendritic cells and triggers innate immune responses
Abstract
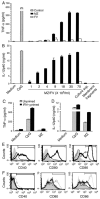
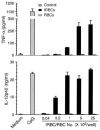
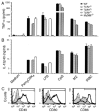
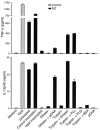
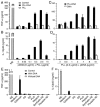
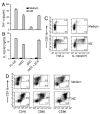
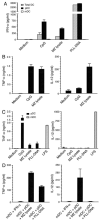
Dendritic cells (DCs) play a crucial role in the development of protective immunity to malaria. However, it remains unclear how malaria parasites trigger immune responses in DCs. In this study, we purified merozoites, food vacuoles, and parasite membrane fragments released during the Plasmodium falciparum schizont burst to homogeneity and tested for the activation of bone marrow-derived DCs from wild-type and TLR2(-/-), TLR4(-/-), TLR9(-/-), and MyD88(-/-) C57BL/6J mice. The results demonstrate that a protein-DNA complex is the exclusive parasite component that activates DCs by a TLR9-dependent pathway to produce inflammatory cytokines. Complex formation with proteins is essential for the entry of parasite DNA into DCs for TLR9 recognition and, thus, proteins convert inactive DNA into a potent immunostimulatory molecule. Exogenous cationic polymers, polylysine and chitosan, can impart stimulatory activity to parasite DNA, indicating that complex formation involves ionic interactions. Merozoites and DNA-protein complex could also induce inflammatory cytokine responses in human blood DCs. Hemozoin is neither a TLR9 ligand for DCs nor functions as a carrier of DNA into cells. Additionally, although TLR9 is critical for DCs to induce the production of IFN-gamma by NK cells, this receptor is not required for NK cells to secret IFN-gamma, and cell-cell contact among myeloid DCs, plasmacytoid DCs, and NK cells is required for IFN-gamma production. Together, these results contribute substantially toward the understanding of malaria parasite-recognition mechanisms. More importantly, our finding that proteins and carbohydrate polymers are able to confer stimulatory activity to an otherwise inactive parasite DNA have important implications for the development of a vaccine against malaria.
Figures
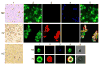

Comment in
-
How do malaria parasites activate dendritic cells?Future Microbiol. 2010 Aug;5(8):1167-71. doi: 10.2217/fmb.10.85. Future Microbiol. 2010. PMID: 20722596
Similar articles
-
The nucleosome (histone-DNA complex) is the TLR9-specific immunostimulatory component of Plasmodium falciparum that activates DCs.PLoS One. 2011;6(6):e20398. doi: 10.1371/journal.pone.0020398. Epub 2011 Jun 8. PLoS One. 2011. PMID: 21687712 Free PMC article.
-
Malaria blood stage parasites activate human plasmacytoid dendritic cells and murine dendritic cells through a Toll-like receptor 9-dependent pathway.J Immunol. 2004 Apr 15;172(8):4926-33. doi: 10.4049/jimmunol.172.8.4926. J Immunol. 2004. PMID: 15067072
-
CD36 contributes to malaria parasite-induced pro-inflammatory cytokine production and NK and T cell activation by dendritic cells.PLoS One. 2013 Oct 28;8(10):e77604. doi: 10.1371/journal.pone.0077604. eCollection 2013. PLoS One. 2013. PMID: 24204889 Free PMC article.
-
Parasite Recognition and Signaling Mechanisms in Innate Immune Responses to Malaria.Front Immunol. 2018 Dec 19;9:3006. doi: 10.3389/fimmu.2018.03006. eCollection 2018. Front Immunol. 2018. PMID: 30619355 Free PMC article. Review.
-
Antigen presentation and dendritic cell biology in malaria.Parasite Immunol. 2006 Jan-Feb;28(1-2):5-14. doi: 10.1111/j.1365-3024.2006.00772.x. Parasite Immunol. 2006. PMID: 16438671 Review.
Cited by
-
RTP4 inhibits IFN-I response and enhances experimental cerebral malaria and neuropathology.Proc Natl Acad Sci U S A. 2020 Aug 11;117(32):19465-19474. doi: 10.1073/pnas.2006492117. Epub 2020 Jul 24. Proc Natl Acad Sci U S A. 2020. PMID: 32709745 Free PMC article.
-
Opsonization of malaria-infected erythrocytes activates the inflammasome and enhances inflammatory cytokine secretion by human macrophages.Malar J. 2012 Oct 9;11:343. doi: 10.1186/1475-2875-11-343. Malar J. 2012. PMID: 23046548 Free PMC article.
-
Chronic TLR7 and TLR9 signaling drives anemia via differentiation of specialized hemophagocytes.Science. 2019 Jan 11;363(6423):eaao5213. doi: 10.1126/science.aao5213. Science. 2019. PMID: 30630901 Free PMC article.
-
Innate immunity to malaria-The role of monocytes.Immunol Rev. 2020 Jan;293(1):8-24. doi: 10.1111/imr.12830. Epub 2019 Dec 16. Immunol Rev. 2020. PMID: 31840836 Free PMC article. Review.
-
Dual engagement of the NLRP3 and AIM2 inflammasomes by plasmodium-derived hemozoin and DNA during malaria.Cell Rep. 2014 Jan 16;6(1):196-210. doi: 10.1016/j.celrep.2013.12.014. Epub 2014 Jan 2. Cell Rep. 2014. PMID: 24388751 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

