Diversity of Micrurus snake species related to their venom toxic effects and the prospective of antivenom neutralization
- PMID: 20231886
- PMCID: PMC2834742
- DOI: 10.1371/journal.pntd.0000622
Diversity of Micrurus snake species related to their venom toxic effects and the prospective of antivenom neutralization
Abstract
Background: Micrurus snake bites can cause death by muscle paralysis and respiratory arrest, few hours after envenomation. The specific treatment for coral snake envenomation is the intravenous application of heterologous antivenom and, in Brazil, it is produced by horse immunization with a mixture of M. corallinus and M. frontalis venoms, snakes that inhabit the South and Southeastern regions of the country. However, this antivenom might be inefficient, considering the existence of intra- and inter-specific variations in the composition of the venoms. Therefore, the aim of the present study was to investigate the toxic properties of venoms from nine species of Micrurus: eight present in different geographic regions of Brazil (M. frontalis, M. corallinus, M. hemprichii, M. spixii, M. altirostris, M. surinamensis, M. ibiboboca, M. lemniscatus) and one (M. fulvius) with large distribution in Southeastern United States and Mexico. This study also analyzed the antigenic cross-reactivity and the neutralizing potential of the Brazilian coral snake antivenom against these Micrurus venoms.
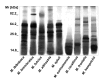
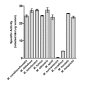
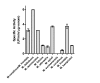
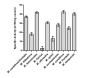
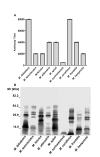
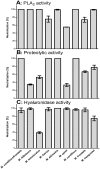
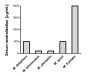
Methodology/principal findings: Analysis of protein composition and toxicity revealed a large diversity of venoms from the nine Micrurus species. ELISA and Western blot assays showed a varied capability of the therapeutic antivenom to recognize the diverse species venom components. In vivo and in vitro neutralization assays indicated that the antivenom is not able to fully neutralize the toxic activities of all venoms.
Conclusion: These results indicate the existence of a large range of both qualitative and quantitative variations in Micrurus venoms, probably reflecting the adaptation of the snakes from this genus to vastly dissimilar habitats. The data also show that the antivenom used for human therapy in Brazil is not fully able to neutralize the main toxic activities present in the venoms from all Micrurus species occurring in the country. It suggests that modifications in the immunization scheme, with the inclusion of other venoms in the antigenic mixture, should occur in order to generate effective therapeutic coral snake antivenom.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Campbell JA, Lamar WW. 2004. The venomous reptiles of the western hemisphere. 2. ed., Ithaca, New York, Cornell University Press.
-
- Roze JA. A checklist of the New World venomous coral snakes (Elapidae), with description of new forms. Am Mus Novitates. 1967;2287:1–60.
-
- Roze JA. New World coral snake (Elapidae): a toxonomic and biological summary. Mem Inst Butantan. 1983;46:305–338.
-
- Roze JA, Bernal-Carlo A. Las serpientes corales venenosas del genero Leptomicrurus (Serpentes, Elapidae) de Sudamérica con descripción de una nueva subespecie. Boll Mus Sci Nat Torino. 1987;5:573–608.
-
- Roze JA. Coral snakes of the Americans. Biology, identification, and venoms. Krieger Publishing Co Florida 262. 1996.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

