Early embryonic chromosome instability results in stable mosaic pattern in human tissues
- PMID: 20231887
- PMCID: PMC2834743
- DOI: 10.1371/journal.pone.0009591
Early embryonic chromosome instability results in stable mosaic pattern in human tissues
Abstract
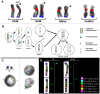
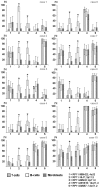
The discovery of copy number variations (CNV) in the human genome opened new perspectives on the study of the genetic causes of inherited disorders and the aetiology of common diseases. Here, a single-cell-level investigation of CNV in different human tissues led us to uncover the phenomenon of mitotically derived genomic mosaicism, which is stable in different cell types of one individual. The CNV mosaic ratios were different between the 10 individuals studied. However, they were stable in the T lymphocytes, immortalized B lymphoblastoid cells, and skin fibroblasts analyzed in each individual. Because these cell types have a common origin in the connective tissues, we suggest that mitotic changes in CNV regions may happen early during embryonic development and occur only once, after which the stable mosaic ratio is maintained throughout the differentiated tissues. This concept is further supported by a unique study of immortalized B lymphoblastoid cell lines obtained with 20 year difference from two subjects. We provide the first evidence of somatic mosaicism for CNV, with stable variation ratios in different cell types of one individual leading to the hypothesis of early embryonic chromosome instability resulting in stable mosaic pattern in human tissues. This concept has the potential to open new perspectives in personalized genetic diagnostics and can explain genetic phenomena like diminished penetrance in autosomal dominant diseases. We propose that further genomic studies should focus on the single-cell level, to better understand the aetiology of aging and diseases mediated by somatic mutations.
Conflict of interest statement
Figures

Similar articles
-
Somatic mosaicism for copy-neutral loss of heterozygosity and DNA copy number variations in the human genome.BMC Genomics. 2015 Sep 16;16(1):703. doi: 10.1186/s12864-015-1916-3. BMC Genomics. 2015. PMID: 26376747 Free PMC article.
-
The human genome puzzle - the role of copy number variation in somatic mosaicism.Curr Genomics. 2010 Sep;11(6):426-31. doi: 10.2174/138920210793176047. Curr Genomics. 2010. PMID: 21358987 Free PMC article.
-
The Cytogenomic "Theory of Everything": Chromohelkosis May Underlie Chromosomal Instability and Mosaicism in Disease and Aging.Int J Mol Sci. 2020 Nov 6;21(21):8328. doi: 10.3390/ijms21218328. Int J Mol Sci. 2020. PMID: 33171981 Free PMC article.
-
Clinical, cytogenetic, and molecular analyses of prenatally diagnosed mosaic tetrasomy for distal chromosome 15q and review of the literature.Prenat Diagn. 2004 Oct;24(10):767-73. doi: 10.1002/pd.977. Prenat Diagn. 2004. PMID: 15503270 Review.
-
The cytogenetic constitution of human blastocysts: insights from comprehensive chromosome screening strategies.Hum Reprod Update. 2019 Jan 1;25(1):15-33. doi: 10.1093/humupd/dmy036. Hum Reprod Update. 2019. PMID: 30395265 Review.
Cited by
-
Single cell genomics of the brain: focus on neuronal diversity and neuropsychiatric diseases.Curr Genomics. 2012 Sep;13(6):477-88. doi: 10.2174/138920212802510439. Curr Genomics. 2012. PMID: 23449087 Free PMC article.
-
Hydroxyurea induces de novo copy number variants in human cells.Proc Natl Acad Sci U S A. 2011 Oct 18;108(42):17360-5. doi: 10.1073/pnas.1109272108. Epub 2011 Oct 10. Proc Natl Acad Sci U S A. 2011. PMID: 21987784 Free PMC article.
-
Somatic genome variations in health and disease.Curr Genomics. 2010 Sep;11(6):387-96. doi: 10.2174/138920210793176065. Curr Genomics. 2010. PMID: 21358982 Free PMC article.
-
Somatic mosaicism for copy-neutral loss of heterozygosity and DNA copy number variations in the human genome.BMC Genomics. 2015 Sep 16;16(1):703. doi: 10.1186/s12864-015-1916-3. BMC Genomics. 2015. PMID: 26376747 Free PMC article.
-
Influence of aflatoxin B1 on copy number variants in human leukocytes in vitro.Mol Cytogenet. 2015 Apr 9;8:25. doi: 10.1186/s13039-015-0131-x. eCollection 2015. Mol Cytogenet. 2015. PMID: 25901182 Free PMC article.
References
-
- Freeman J, Perry G, Feuk L, Redon R, McCarroll S, et al. Copy number variation: New insights in genome diversity. Genome Res. 2006;16:949–961. - PubMed
-
- Weise A, Gross M, Mrasek K, Mkrtchyan H, Horsthemke B, et al. Parental-origin-determination fluorescence in situ hybridization distinguishes homologous human chromosomes on a single-cell level. Int J Mol Med. 2008;21:189–200. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

