Phospholipase D1 mediates AMP-activated protein kinase signaling for glucose uptake
- PMID: 20231899
- PMCID: PMC2834755
- DOI: 10.1371/journal.pone.0009600
Phospholipase D1 mediates AMP-activated protein kinase signaling for glucose uptake
Abstract
Background: Glucose homeostasis is maintained by a balance between hepatic glucose production and peripheral glucose utilization. In skeletal muscle cells, glucose utilization is primarily regulated by glucose uptake. Deprivation of cellular energy induces the activation of regulatory proteins and thus glucose uptake. AMP-activated protein kinase (AMPK) is known to play a significant role in the regulation of energy balances. However, the mechanisms related to the AMPK-mediated control of glucose uptake have yet to be elucidated.
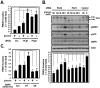
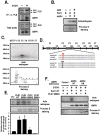
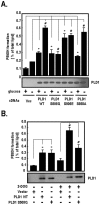
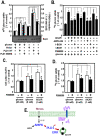
Methodology/principal findings: Here, we found that AMPK-induced phospholipase D1 (PLD1) activation is required for (14)C-glucose uptake in muscle cells under glucose deprivation conditions. PLD1 activity rather than PLD2 activity is significantly enhanced by glucose deprivation. AMPK-wild type (WT) stimulates PLD activity, while AMPK-dominant negative (DN) inhibits it. AMPK regulates PLD1 activity through phosphorylation of the Ser-505 and this phosphorylation is increased by the presence of AMP. Furthermore, PLD1-S505Q, a phosphorylation-deficient mutant, shows no changes in activity in response to glucose deprivation and does not show a significant increase in (14)C-glucose uptake when compared to PLD1-WT. Taken together, these results suggest that phosphorylation of PLD1 is important for the regulation of (14)C-glucose uptake. In addition, extracellular signal-regulated kinase (ERK) is stimulated by AMPK-induced PLD1 activation through the formation of phosphatidic acid (PA), which is a product of PLD. An ERK pharmacological inhibitor, PD98059, and the PLD inhibitor, 1-BtOH, both attenuate (14)C-glucose uptake in muscle cells. Finally, the extracellular stresses caused by glucose deprivation or aminoimidazole carboxamide ribonucleotide (AICAR; AMPK activator) regulate (14)C-glucose uptake and cell surface glucose transport (GLUT) 4 through ERK stimulation by AMPK-mediated PLD1 activation.
Conclusions/significance: These results suggest that AMPK-mediated PLD1 activation is required for (14)C-glucose uptake through ERK stimulation. We propose that the AMPK-mediated PLD1 pathway may provide crucial clues to understanding the mechanisms involved in glucose uptake.
Conflict of interest statement
Figures

References
-
- Barnes BR, Zierath JR. Role of AMP–activated protein kinase in the control of glucose homeostasis. Curr Mol Med. 2005;5:341–348. - PubMed
-
- Kahn BB, Alquier T, Carling D, Hardie DG. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 2005;1:15–25. - PubMed
-
- Musi N, Yu H, Goodyear LJ. AMP-activated protein kinase regulation and action in skeletal muscle during exercise. Biochem Soc Trans. 2003;31:191–195. - PubMed
-
- Itani SI, Saha AK, Kurowski TG, Coffin HR, Tornheim K, et al. Glucose autoregulates its uptake in skeletal muscle: involvement of AMP-activated protein kinase. Diabetes. 2003;52:1635–1640. - PubMed
-
- Mu J, Jr, Brozinick JT, Valladaress O, Bucan M, Birnbaum MJ. A role for AMP-activated protein kinase in contraction- and hypoxia-regulated glucose transport in skeletal muscle. Mol Cell. 2001;7:1085–1094. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

