Interspecies scaling of receptor-mediated pharmacokinetics and pharmacodynamics of type I interferons
- PMID: 20232116
- PMCID: PMC3176922
- DOI: 10.1007/s11095-010-0098-6
Interspecies scaling of receptor-mediated pharmacokinetics and pharmacodynamics of type I interferons
Abstract
Purpose: To develop an integrated mechanism-based modeling approach for the interspecies scaling of pharmacokinetic (PK) and pharmacodynamic (PD) properties of type I interferons (IFNs) that exhibit target-mediated drug disposition (TMDD).
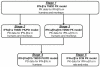
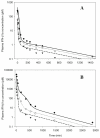
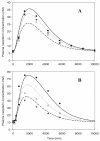
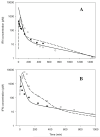
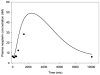
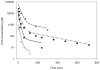
Methods: PK and PD profiles of human IFN-beta1a, IFN-beta1b, and IFN-alpha2a in humans, monkeys, rats, and mice from nine studies were extracted from the literature by digitization. Concentration-time profiles from different species were fitted simultaneously using various allometric relationships to scale model-specific parameters.
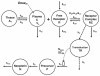
Results: PK/PD profiles of IFN-beta1a in humans and monkeys were successfully characterized by utilizing the same rate constant parameters and scaling the volume of the central compartment to body weight using an allometric exponent of 1. Concentration and effect profiles of other IFNs were also well described by changing only the affinity of the drug to its receptor. PK profiles in rodents were simulated using an allometric exponent of -0.25 for the first-order elimination rate constant, and no receptor-binding was included given the lack of cross-reactivity.
Conclusions: An integrated TMDD PK/PD model was successfully combined with classic allometric scaling techniques and showed good predictive performance. Several parameters obtained from one IFN can be effectively shared to predict the kinetic behavior of other IFN subtypes.
Figures

Similar articles
-
Interspecies scaling of interferon disposition and comparison of allometric scaling with concentration-time transformations.J Pharm Sci. 1995 Nov;84(11):1285-90. doi: 10.1002/jps.2600841106. J Pharm Sci. 1995. PMID: 8587044 Clinical Trial.
-
Type I interferon receptor is a primary regulator of target-mediated drug disposition of interferon-beta in mice.J Pharmacol Exp Ther. 2010 Jul;334(1):327-32. doi: 10.1124/jpet.110.167650. Epub 2010 Apr 20. J Pharmacol Exp Ther. 2010. PMID: 20406858 Free PMC article.
-
Quantitative prediction of human pharmacokinetics and pharmacodynamics of imigliptin, a novel DPP-4 inhibitor, using allometric scaling, IVIVE and PK/PD modeling methods.Eur J Pharm Sci. 2016 Jun 30;89:73-82. doi: 10.1016/j.ejps.2016.04.020. Epub 2016 Apr 21. Eur J Pharm Sci. 2016. PMID: 27108678
-
Interspecies allometric analysis of the comparative pharmacokinetics of 44 drugs across veterinary and laboratory animal species.J Vet Pharmacol Ther. 1997 Dec;20(6):453-63. doi: 10.1046/j.1365-2885.1997.00095.x. J Vet Pharmacol Ther. 1997. PMID: 9430769 Review.
-
Pegylated interferon with ribavirin therapy for chronic infection with the hepatitis C virus.Expert Opin Pharmacother. 2003 May;4(5):685-91. doi: 10.1517/14656566.4.5.685. Expert Opin Pharmacother. 2003. PMID: 12739994 Review.
Cited by
-
Sustained plasma hepcidin suppression and iron elevation by Anticalin-derived hepcidin antagonist in cynomolgus monkey.Br J Pharmacol. 2018 Apr;175(7):1054-1065. doi: 10.1111/bph.14143. Epub 2018 Feb 23. Br J Pharmacol. 2018. PMID: 29329501 Free PMC article.
-
Interspecies modeling and prediction of human exenatide pharmacokinetics.Pharm Res. 2013 Mar;30(3):751-60. doi: 10.1007/s11095-012-0917-z. Epub 2012 Nov 15. Pharm Res. 2013. PMID: 23229855 Free PMC article.
-
Pharmacokinetics and toxicology of therapeutic proteins: Advances and challenges.World J Biol Chem. 2012 Apr 26;3(4):73-92. doi: 10.4331/wjbc.v3.i4.73. World J Biol Chem. 2012. PMID: 22558487 Free PMC article.
-
Scaling Pharmacodynamics from Rats to Humans to Support Erythropoietin and Romiplostim Combination Therapy to Treat Erythropoietin-Resistant Anemia.Pharmaceutics. 2023 Jan 19;15(2):344. doi: 10.3390/pharmaceutics15020344. Pharmaceutics. 2023. PMID: 36839666 Free PMC article.
-
Pharmacokinetic-pharmacodynamic modelling of the anti-FcRn monoclonal antibody rozanolixizumab: Translation from preclinical stages to the clinic.CPT Pharmacometrics Syst Pharmacol. 2022 Jan;11(1):116-128. doi: 10.1002/psp4.12739. Epub 2021 Nov 23. CPT Pharmacometrics Syst Pharmacol. 2022. PMID: 34735735 Free PMC article.
References
-
- Bekisz J, Schmeisser H, Hernandez J, Goldman ND, Zoon KC. Human interferons alpha, beta and omega. Growth Factors. 2004;22:243–51. - PubMed
-
- Samarajiwa SA, Wilson W, Hertzog PJ, Interferons TI. Genetics and structure. In: Meager A, editor. The interferons: characterization and application. WILEY-VCH Verlag GmbH & Co. KGaA; Weinheim: 2006. pp. 3–34.
-
- Mogensen KE, Lewerenz M, Reboul J, Lutfalla G, Uze G. The type I interferon receptor: structure, function, and evolution of a family business. J Interferon Cytokine Res. 1999;19:1069–98. - PubMed
-
- de Weerd NA, Samarajiwa SA, Hertzog PJ. Type I interferon receptors: biochemistry and biological functions. J Biol Chem. 2007;282:20053–7. - PubMed
-
- Pestka S, Langer JA, Zoon KC, Samuel CE. Interferons and their actions. Annu Rev Biochem. 1987;56:727–77. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

