Prolonged blockade of VEGF receptors does not damage retinal photoreceptors or ganglion cells
- PMID: 20232317
- PMCID: PMC4005719
- DOI: 10.1002/jcp.22129
Prolonged blockade of VEGF receptors does not damage retinal photoreceptors or ganglion cells
Abstract
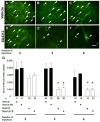
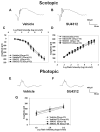
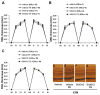
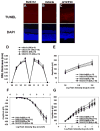
It has recently been reported that relatively short-term inhibition of vascular endothelial growth factor (VEGF) signaling can cause photoreceptor cell death, a potentially clinically important finding since VEGF blockade has become an important modality of treatment of ocular neovascularization and macular edema. However, in a set of studies in which we achieved extended and complete blockage of VEGF-induced vascular leakage through retinal expression of a VEGF binding protein, we did not observe any toxicity to retinal neurons. To follow-up on these apparently discrepant findings, we designed a set of experiments with the kinase inhibitor SU4312, which blocks phosphorylation of VEGF receptors, to look directly for evidence of VEGF inhibition-related retinal toxicity. Using transgenic mice with sustained expression of VEGF in photoreceptors, we determined that periocular injection of 3 microg of SU4312 every 5 days markedly suppressed subretinal neovascularization, indicating effective blockade of VEGF signaling. Wild-type mice given periocular injections of 5 microg of SU4312 every 5 days for up to 12 weeks showed normal scotopic and photopic electroretinograms (ERGs), no TUNEL stained cells in the retina, and no reduction in outer nuclear layer thickness. Incubation of cultured ganglion cells or retinal cultures containing photoreceptors with high doses of SU4312 did not reduce cell viability. These data suggest that blocking VEGF signaling in the retina for up to 12 weeks does not damage photoreceptors nor alter ERG function and should reassure patients who are receiving frequent injections of VEGF antagonists for choroidal and retinal vascular diseases.
(c) 2010 Wiley-Liss, Inc.
Figures



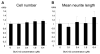
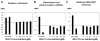
Similar articles
-
Endogenous VEGF is required for visual function: evidence for a survival role on müller cells and photoreceptors.PLoS One. 2008;3(11):e3554. doi: 10.1371/journal.pone.0003554. Epub 2008 Nov 3. PLoS One. 2008. PMID: 18978936 Free PMC article.
-
Comparative toxicity and proliferation testing of aflibercept, bevacizumab and ranibizumab on different ocular cells.Br J Ophthalmol. 2013 Jul;97(7):917-23. doi: 10.1136/bjophthalmol-2013-303130. Epub 2013 May 17. Br J Ophthalmol. 2013. PMID: 23686000
-
Ruboxistaurin, a PKCbeta inhibitor, inhibits retinal neovascularization via suppression of phosphorylation of ERK1/2 and Akt.Exp Eye Res. 2010 Jan;90(1):137-45. doi: 10.1016/j.exer.2009.09.022. Epub 2009 Oct 13. Exp Eye Res. 2010. PMID: 19825373
-
Silver nano - a trove for retinal therapies.J Control Release. 2010 Jul 14;145(2):76-90. doi: 10.1016/j.jconrel.2010.03.022. Epub 2010 Mar 29. J Control Release. 2010. PMID: 20359511 Review.
-
[Aging and retinal vascular diseases].Nippon Ganka Gakkai Zasshi. 2007 Mar;111(3):207-30; discussion 231. Nippon Ganka Gakkai Zasshi. 2007. PMID: 17402563 Review. Japanese.
Cited by
-
Lentiviral delivered aflibercept OXB-203 for treatment of neovascular AMD.Mol Ther Methods Clin Dev. 2023 Jul 15;30:350-366. doi: 10.1016/j.omtm.2023.07.001. eCollection 2023 Sep 14. Mol Ther Methods Clin Dev. 2023. PMID: 37637380 Free PMC article.
-
Comparison of the efficacy of aflibercept, ranibizumab, and bevacizumab in an RPE/choroid organ culture.Graefes Arch Clin Exp Ophthalmol. 2014 Oct;252(10):1593-8. doi: 10.1007/s00417-014-2719-y. Epub 2014 Jul 22. Graefes Arch Clin Exp Ophthalmol. 2014. PMID: 25047874
-
Tyrosine kinase blocking collagen IV-derived peptide suppresses ocular neovascularization and vascular leakage.Sci Transl Med. 2017 Jan 18;9(373):eaai8030. doi: 10.1126/scitranslmed.aai8030. Sci Transl Med. 2017. PMID: 28100839 Free PMC article.
-
The anti-cancer agent SU4312 unexpectedly protects against MPP(+) -induced neurotoxicity via selective and direct inhibition of neuronal NOS.Br J Pharmacol. 2013 Mar;168(5):1201-14. doi: 10.1111/bph.12004. Br J Pharmacol. 2013. PMID: 23062100 Free PMC article.
-
Long-Term Safety Evaluation of Continuous Intraocular Delivery of Aflibercept by the Intravitreal Gene Therapy Candidate ADVM-022 in Nonhuman Primates.Transl Vis Sci Technol. 2021 Jan 29;10(1):34. doi: 10.1167/tvst.10.1.34. eCollection 2021 Jan. Transl Vis Sci Technol. 2021. PMID: 33532145 Free PMC article.
References
-
- Barres BA, Chun Linda LY. Purification of rat retinal ganglion cells by immunopanning. Neuroprotocols. 1993;2:201–204.
-
- Bocker-Meffert S, Rowenstiel P, Rohl C, Warneke N, Held-Feindt J, Sievers J, Lucius R. Erythropoietin and VEGF promote neural outgrowth from retinal explants in postnatal rats. Invest Ophthalmol Vis Sci. 2002;43:2021–2026. - PubMed
-
- Bonnet D, Garcia M, Vecino E, Lorentz JG, Sahel J, Hicks D. Brain-derived neurotrophic factor signalling in adult pig retinal ganglion cell neurite regeneration in vitro. Brain Res. 2004;1007:142–151. - PubMed
-
- Brown DM, Kaiser PK, Michels M, Soubrane G, Heier JS, Kim RY, Sy JP, Schneider S the Anchor Study Group. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1432–1444. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

