Water maze experience and prenatal choline supplementation differentially promote long-term hippocampal recovery from seizures in adulthood
- PMID: 20232399
- PMCID: PMC2972409
- DOI: 10.1002/hipo.20783
Water maze experience and prenatal choline supplementation differentially promote long-term hippocampal recovery from seizures in adulthood
Abstract
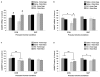
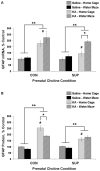
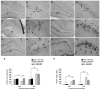
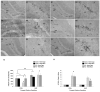
Status epilepticus (SE) in adulthood dramatically alters the hippocampus and produces spatial learning and memory deficits. Some factors, like environmental enrichment and exercise, may promote functional recovery from SE. Prenatal choline supplementation (SUP) also protects against spatial memory deficits observed shortly after SE in adulthood, and we have previously reported that SUP attenuates the neuropathological response to SE in the adult hippocampus just 16 days after SE. It is unknown whether SUP can ameliorate longer-term cognitive and neuropathological consequences of SE, whether repeatedly engaging the injured hippocampus in a cognitive task might facilitate recovery from SE, and whether our prophylactic prenatal dietary treatment would enable the injured hippocampus to more effectively benefit from cognitive rehabilitation. To address these issues, adult offspring from rat dams that received either a control (CON) or SUP diet on embryonic days 12-17 first received training on a place learning water maze task (WM) and were then administered saline or kainic acid (KA) to induce SE. Rats then either remained in their home cage, or received three additional WM sessions at 3, 6.5, and 10 weeks after SE to test spatial learning and memory retention. Eleven weeks after SE, the brains were analyzed for several hippocampal markers known to be altered by SE. SUP attenuated SE-induced spatial learning deficits and completely rescued spatial memory retention by 10 weeks post-SE. Repeated WM experience prevented SE-induced declines in glutamic acid decarboxylase (GAD) and dentate gyrus neurogenesis, and attenuated increased glial fibrilary acidic protein (GFAP) levels. Remarkably, SUP alone was similarly protective to an even greater extent, and SUP rats that were water maze trained after SE showed reduced hilar migration of newborn neurons. These findings suggest that prophylactic SUP is protective against the long-term cognitive and neuropathological effects of KA-induced SE, and that rehabilitative cognitive enrichment may be partially beneficial.
Copyright © 2010 Wiley-Liss, Inc.
Figures





Similar articles
-
Prenatal choline supplementation attenuates neuropathological response to status epilepticus in the adult rat hippocampus.Neurobiol Dis. 2008 May;30(2):255-69. doi: 10.1016/j.nbd.2008.01.008. Epub 2008 Feb 16. Neurobiol Dis. 2008. PMID: 18353663 Free PMC article.
-
Spatial memory and hippocampal plasticity are differentially sensitive to the availability of choline in adulthood as a function of choline supply in utero.Brain Res. 2008 Oct 27;1237:153-66. doi: 10.1016/j.brainres.2008.08.074. Epub 2008 Sep 4. Brain Res. 2008. PMID: 18778697 Free PMC article.
-
Seizure-induced memory impairment is reduced by choline supplementation before or after status epilepticus.Epilepsy Res. 2002 Jan;48(1-2):3-13. doi: 10.1016/s0920-1211(01)00321-7. Epilepsy Res. 2002. PMID: 11823105
-
Maternal choline supplementation improves spatial learning and adult hippocampal neurogenesis in the Ts65Dn mouse model of Down syndrome.Neurobiol Dis. 2013 Oct;58:92-101. doi: 10.1016/j.nbd.2013.04.016. Epub 2013 Apr 30. Neurobiol Dis. 2013. PMID: 23643842 Free PMC article.
-
A meta-analysis of animal studies on disruption of spatial navigation by prenatal cocaine exposure.Neurotoxicol Teratol. 2007 Sep-Oct;29(5):570-7. doi: 10.1016/j.ntt.2007.06.003. Epub 2007 Jun 30. Neurotoxicol Teratol. 2007. PMID: 17683902 Free PMC article. Review.
Cited by
-
Voluntary running prevents progressive memory decline and increases adult hippocampal neurogenesis and growth factor expression after whole-brain irradiation.Cancer Res. 2010 Nov 15;70(22):9329-38. doi: 10.1158/0008-5472.CAN-10-1854. Epub 2010 Sep 30. Cancer Res. 2010. PMID: 20884629 Free PMC article.
-
Choline nutrition programs brain development via DNA and histone methylation.Cent Nerv Syst Agents Med Chem. 2012 Jun;12(2):82-94. doi: 10.2174/187152412800792706. Cent Nerv Syst Agents Med Chem. 2012. PMID: 22483275 Free PMC article. Review.
-
Perinatal Choline Supplementation Reduces Amyloidosis and Increases Choline Acetyltransferase Expression in the Hippocampus of the APPswePS1dE9 Alzheimer's Disease Model Mice.PLoS One. 2017 Jan 19;12(1):e0170450. doi: 10.1371/journal.pone.0170450. eCollection 2017. PLoS One. 2017. PMID: 28103298 Free PMC article.
-
Maternal choline supplementation protects against age-associated cholinergic and GABAergic basal forebrain neuron degeneration in the Ts65Dn mouse model of Down syndrome and Alzheimer's disease.Neurobiol Dis. 2023 Nov;188:106332. doi: 10.1016/j.nbd.2023.106332. Epub 2023 Oct 26. Neurobiol Dis. 2023. PMID: 37890559 Free PMC article.
-
Choline supplementation in early life improves and low levels of choline can impair outcomes in a mouse model of Alzheimer's disease.Elife. 2024 Jun 21;12:RP89889. doi: 10.7554/eLife.89889. Elife. 2024. PMID: 38904658 Free PMC article.
References
-
- Albright CD, Tsai AY, Friedrich CB, Mar MH, Zeisel SH. Choline availability alters embryonic development of the hippocampus and septum in the rat. Brain Res Dev Brain Res. 1999;113:13–20. - PubMed
-
- Ambrogini P, Orsini L, Mancini C, Ferri P, Ciaroni S, Cuppini R. Learning may reduce neurogenesis in adult rat dentate gyrus. Neurosci Lett. 2004;359:13–16. - PubMed
-
- Arida RM, Scorza FA, Scorza CA, Cavalheiro EA. Is physical activity beneficial for recovery in temporal lobe epilepsy? Evidences from animal studies. Neurosci Biobehav Rev. 2009;33:422–431. - PubMed
-
- Aronica E, van Vliet EA, Mayboroda OA, Troost D, da Silva FH, Gorter JA. Upregulation of metabotropic glutamate receptor subtype mGluR3 and mGluR5 in reactive astrocytes in a rat model of mesial temporal lobe epilepsy. Eur J Neurosci. 2000;12:2333–2344. - PubMed
-
- Ben-Ari Y, Represa A. Brief seizure episodes induce long-term potentiation and mossy fibre sprouting in the hippocampus. Trends Neurosci. 1990;13:312–318. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

