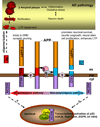
An overview of APP processing enzymes and products
- PMID: 20232515
- PMCID: PMC2889200
- DOI: 10.1007/s12017-009-8104-z
An overview of APP processing enzymes and products
Abstract
The generation of amyloid beta-peptide (A beta) by enzymatic cleavages of the beta-amyloid precursor protein (APP) has been at the center of Alzheimer's disease (AD) research. While the basic process of beta- and gamma-secretase-mediated generation of A beta is text book knowledge, new aspects of A beta and other cleavage products have emerged in recent years. Also our understanding of the enzymes involved in APP proteolysis has increased dramatically. All of these discoveries contribute to a more complete understanding of APP processing and the physiologic and pathologic roles of its secreted and intracellular protein products. Understanding APP processing is important for any therapeutic strategy aimed at reducing A beta levels in AD. In this review, we provide a concise description of the current state of understanding the enzymes involved in APP processing, the cleavage products generated by different processing patterns, and the potential functions of those cleavage products.
Figures
References
-
- Asai M, Hattori C, Szabó B, Sasagawa N, Maruyama K, Tanuma S, Ishiura S. Putative function of ADAM9, ADAM10, and ADAM17 as APP alpha-secretase. Biochem. Biophys. Res. Commun. 2003;301:231–235. - PubMed
-
- Baier M, Apelt J, Riemer C, Gültner S, Schwarz A, Bamme T, Burwinkel M, Schliebs R. Prion infection of mice transgenic for human APPSwe: increased accumulation of cortical formic acid extractable Abeta(1–42) and rapid scrapie disease development. Int. J. Dev. Neurosci. 2008;26:821–824. - PubMed
-
- Böhme L, Hoffmann T, Manhart S, Wolf R, Demuth HU. Isoaspartate-containing amyloid precursor protein-derived peptides alter efficacy and specificity of potential beta-secretases. Biol. Chem. 2008;389:1055–1066. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

