Sequence-dependent Kink-and-Slide deformations of nucleosomal DNA facilitated by histone arginines bound in the minor groove
- PMID: 20232937
- PMCID: PMC2987563
- DOI: 10.1080/07391102.2010.10508586
Sequence-dependent Kink-and-Slide deformations of nucleosomal DNA facilitated by histone arginines bound in the minor groove
Abstract
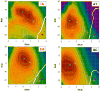
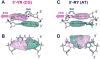
In addition to bending and twisting deformabilities, the lateral displacements of the DNA axis (Kink-and-Slide) play an important role in DNA wrapping around the histone core (M. Y. Tolstorukov, A. V. Colasanti, D. M. McCandlish, W. K. Olson, V. B. Zhurkin, J. Mol. Biol. 371, 725-738 (2007)). Here, we show that these Kink-and-Slide deformations are likely to be stabilized by the arginine residues of histones interacting with the minor groove of DNA. The arginines are positioned asymmetrically in the minor groove, being closer to one strand. The asymmetric arginine-DNA interactions facilitate lateral displacement of base pairs across the DNA grooves, thus leading to a stepwise accumulation of the superhelical pitch of nucleosomal DNA. To understand the sequence dependence of such Kink-and-Slide deformations, we performed all-atom calculations of DNA hexamers with the YR and RY steps in the center. We found that when the unrestrained DNA deformations are allowed, the YR steps tend to bend into the major groove, and RY steps bend into the minor groove. However, when the nucleosomal Kink-and-Slide deformation is considered, the YR steps prove to be more favorable for bending into the minor groove. Overall, the Kink-and-Slide deformation energy of DNA increases in the order TA < CA < CG < GC < AC < AT. We propose a simple stereochemical model accounting for this sequence dependence. Our results agree with experimental data indicating that the TA step most frequently occurs in the minor-groove kink positions in the most stable nucleosomes. Our computations demonstrate that the Kink-and-Slide distortion is accompanied by the BI to BII transition. This fact, together with irregularities in the two-dimensional (Roll, Slide) energy contour maps, suggest that the Kink-and-Slide deformations represent a nonharmonic behavior of the duplex. This explains the difference between the two estimates of the DNA deformation energy in nucleosome - the earlier one made using knowledge-based elastic energy functions, and the current one based on all-atom calculations. Our findings are useful for refining the score functions for the prediction of nucleosome positioning. In addition, the reverse bending behavior of the YR and RY steps revealed under the Kink-and-Slide constraint is important for understanding the molecular mechanisms of binding transcription factors (such as p53) to DNA exposed on the surface of nucleosome.
Figures



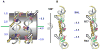



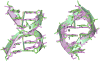
Similar articles
-
Working the kinks out of nucleosomal DNA.Curr Opin Struct Biol. 2011 Jun;21(3):348-57. doi: 10.1016/j.sbi.2011.03.006. Epub 2011 Apr 7. Curr Opin Struct Biol. 2011. PMID: 21482100 Free PMC article. Review.
-
Structure-based analysis of DNA sequence patterns guiding nucleosome positioning in vitro.J Biomol Struct Dyn. 2010 Jun;27(6):821-41. doi: 10.1080/073911010010524947. J Biomol Struct Dyn. 2010. PMID: 20232936 Free PMC article.
-
A novel roll-and-slide mechanism of DNA folding in chromatin: implications for nucleosome positioning.J Mol Biol. 2007 Aug 17;371(3):725-38. doi: 10.1016/j.jmb.2007.05.048. Epub 2007 May 24. J Mol Biol. 2007. PMID: 17585938 Free PMC article.
-
Electrostatic interactions between arginines and the minor groove in the nucleosome.J Biomol Struct Dyn. 2010 Jun;27(6):861-6. doi: 10.1080/07391102.2010.10508587. J Biomol Struct Dyn. 2010. PMID: 20232938 Free PMC article.
-
Two distinct modes of protein-induced bending in DNA.J Mol Biol. 1998 Sep 18;282(2):331-43. doi: 10.1006/jmbi.1998.1994. J Mol Biol. 1998. PMID: 9735291 Review.
Cited by
-
Novel nucleosomal particles containing core histones and linker DNA but no histone H1.Nucleic Acids Res. 2016 Jan 29;44(2):573-81. doi: 10.1093/nar/gkv943. Epub 2015 Sep 22. Nucleic Acids Res. 2016. PMID: 26400169 Free PMC article.
-
Functional specificity of a protein-DNA complex mediated by two arginines bound to the minor groove.J Bacteriol. 2012 Sep;194(17):4727-35. doi: 10.1128/JB.00677-12. Epub 2012 Jun 29. J Bacteriol. 2012. PMID: 22753063 Free PMC article.
-
Transcriptional activation of yeast genes disrupts intragenic nucleosome phasing.Nucleic Acids Res. 2012 Nov;40(21):10753-64. doi: 10.1093/nar/gks870. Epub 2012 Sep 24. Nucleic Acids Res. 2012. PMID: 23012262 Free PMC article.
-
Histone N-tails modulate sequence-specific positioning of nucleosomes.J Biol Chem. 2025 Feb;301(2):108138. doi: 10.1016/j.jbc.2024.108138. Epub 2024 Dec 26. J Biol Chem. 2025. PMID: 39732170 Free PMC article.
-
The nucleosome position-encoding WW/SS sequence pattern is depleted in mammalian genes relative to other eukaryotes.Nucleic Acids Res. 2019 Sep 5;47(15):7942-7954. doi: 10.1093/nar/gkz544. Nucleic Acids Res. 2019. PMID: 31216031 Free PMC article.
References
-
- Sekinger EA, Moqtaderi Z, Struhl K. Mol Cell. 2005;18:735–748. - PubMed
-
- Thastrom A, Lowary PT, Widlund HR, Cao H, Kubista M, Widom J. J Mol Biol. 1999;288:213–229. - PubMed
-
- Gabdank I, Barash D, Trifonov EN. J Biomol Struct Dyn. 2009;26:403–411. - PubMed
-
- Davey CA, Sargent DF, Luger K, Maeder AW, Richmond TJ. J Mol Biol. 2002;319:1097–1113. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
