Screening for small molecules' bilayer-modifying potential using a gramicidin-based fluorescence assay
- PMID: 20233091
- PMCID: PMC2929145
- DOI: 10.1089/adt.2009.0250
Screening for small molecules' bilayer-modifying potential using a gramicidin-based fluorescence assay
Abstract
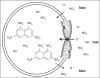
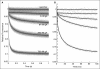
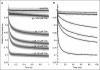
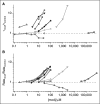
Many drugs and other small molecules used to modulate biological function are amphiphiles that adsorb at the bilayer/solution interface and thereby alter lipid bilayer properties. This is important because membrane proteins are energetically coupled to their host bilayer by hydrophobic interactions. Changes in bilayer properties thus alter membrane protein function, which provides a possible mechanism for "off-target" drug effects. We have previously shown that channels formed by the linear gramicidins are suitable probes for changes in lipid bilayer properties, as experienced by bilayer-spanning proteins. We now report a gramicidin-based fluorescence assay for changes in bilayer properties. The assay is based on measuring the time course of fluorescence quenching in fluorophore-loaded large unilamellar vesicles, due to entry of a gramicidin channel-permeable quencher. The method is scalable and suitable for both mechanistic studies and high-throughput screening for bilayer-perturbing, potential off-target effects, which we illustrate using capsaicin (Cap) and other compounds.
Figures


References
-
- Lipinski CA. Lombardo F. Dominy BW. Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 1997;23:3–26. - PubMed
-
- Leeson PD. Springthorpe B. The influence of drug-like concepts on decision-making in medicinal chemistry. Nat Rev Drug Discov. 2007;6:881–890. - PubMed
-
- Keserü GM. Makara GM. The influence of lead discovery strategies on the properties of drug candidates. Nat Rev Drug Discov. 2009;8:203–212. - PubMed
-
- Waring MJ. Defining optimum lipophilicity and molecular weight ranges for drug candidates-Molecular weight dependent lower logD limits based on permeability. Bioorg Med Chem Lett. 2009;19:2844–2851. - PubMed
-
- Evans E. Rawicz W. Hofmann AF. Lipid bilayer expansion and mechanical disruption in solutions of water-soluble bile acid. In: Hofmann AF, editor; Paumgartner G, editor; Stiehl A, editor. Bile Acids in Gastroenterology: Basic and Clinical Advances. Kluwer Academic Publishers; Dordrecht: 1995. pp. 59–68.
Appendix References
-
- Berberan-Santos MN. Bodunov EN. Valeur B. Mathematical functions for the analysis of luminescence decays with underlying distributions 1. Kohlrausch decay function (stretched exponential) Chem Phys. 2005;315:171–182.
-
- Avdeef A. Physicochemical profiling (solubility, permeability and charge state) Curr Top Med Chem. 2001;1:277–351. - PubMed
-
- Seydel JK. Wiese M. Drug-Membrane Interactions: Analysis, Drug Distribution, Modeling. Wiley-VCH; Weinheim, Germany: 2002.
-
- Søgaard R. Werge TM. Bertelsen C. Lundbye C. Madsen KL. Nielsen CH, et al. GABAA receptor function is regulated by lipid bilayer elasticity. Biochemistry. 2006;45:13118–13129. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

