Pharmacokinetic interactions between ritonavir and quinine in healthy volunteers following concurrent administration
- PMID: 20233197
- PMCID: PMC2829696
- DOI: 10.1111/j.1365-2125.2009.03566.x
Pharmacokinetic interactions between ritonavir and quinine in healthy volunteers following concurrent administration
Erratum in
- Br J Clin Pharmacol. 2010 May;69(5):571
Abstract
Aims: To evaluate the pharmacokinetic interactions between ritonavir and quinine in healthy volunteers.
Methods: Ten healthy volunteers were each given 600-mg single oral doses of quinine alone, ritonavir alone (200 mg every 12 h for 9 days), and quinine in combination with ritonavir, in a three-period pharmacokinetic nonrandomized sequential design study. Quinine was co-administered with the 15th dose of ritonavir. Blood samples collected at predetermined time intervals were analysed for ritonavir, quinine and its major metabolite, 3-hydroxyquinine, using a validated high-performance liquid chromatography method.
Results: Concurrent ritonavir administration resulted in about fourfold increases in both the C(max) and AUC(T)[C(max) 2.79 +/- 0.22 vs. 10.72 +/- 0.32 mg l(-1), 95% confidence interval (CI) 7.81, 8.04; AUC 50.06 +/- 2.52 vs. 220.47 +/- 6.68 mg h(-1) l(-1), 95% CI 166.3, 175.3], a significant increase (P < 0.01) in the elimination half-life (11.15 +/- 0.80 vs. 13.37 +/- 0.33 h, 95% CI 1.64, 2.77) and about a 4.5-fold decrease in CL/F (12.01 +/- 0.61 vs. 2.71 +/- 0.09 l h(-1)) of quinine. Also, with ritonavir, there was a pronounced reduction of AUC(metabolite)/AUC(unchanged drug) ratio of quinine (1.35 +/- 0.10 vs. 0.13 +/- 0.02) along with a marked decrease in C(max) (1.80 +/- 0.12 vs. 0.96 +/- 0.09 mg l(-1)) and AUC(0-48h) (62.80 +/- 6.30 vs. 25.61 +/- 2.44 mg h(-1) l(-1)) of the metabolite. Similarly, quinine caused modest but significant increases (P < 0.01) in the C(max), AUC and elimination T((1/2)) of ritonavir.
Conclusions: Downward dosage adjustment of quinine appears necessary when concurrently administered with ritonavir.
Figures
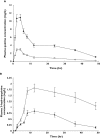
 ); Quinine with Ritonavir (
); Quinine with Ritonavir ( ); (B) 3-hydroxyquinine alone (
); (B) 3-hydroxyquinine alone ( ); 3-hydroxyquinine in presence of ritonavir (
); 3-hydroxyquinine in presence of ritonavir ( )
)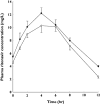
 ); Ritonavir in presence of quinine (
); Ritonavir in presence of quinine ( )
)References
-
- World Health Organization. Malaria and HIV/AIDS interactions and implications: conclusions of a technical consultation convened by WHO. 23–25 June 2004. Available at: http://www.who.int/malaria/malaria_HIV/malaria_hiv_flyer.pdf.
-
- Grinwade K, French N, Mbatha DD, Zungu DD, Dedicoat M, Gilks CF. HIV infection as a cofactor for severe falciparum malaria in adults living in a region of unstable malaria transmission in South Africa. AIDS. 2004;18:547–9. - PubMed
-
- Adam I, Ali DM, Noureldin W, Elbashir MI. Quinine for treatment of chloroquine-resistant falciparum malaria in pregnant and non-pregnant Sudanese women. Ann Trop Med Parasitol. 2005;99:427–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

