Genistein potentiates activity of the cation channel TRPC5 independently of tyrosine kinases
- PMID: 20233211
- PMCID: PMC2850405
- DOI: 10.1111/j.1476-5381.2010.00636.x
Genistein potentiates activity of the cation channel TRPC5 independently of tyrosine kinases
Abstract
Background and purpose: TRPC5 is a Ca(2+)-permeable channel with multiple modes of activation. We have explored the effects of genistein, a plant-derived isoflavone, on TRPC5 activity, and the mechanism(s) involved.
Experimental approach: Effects of genistein on TRPC5 channels were investigated in TRPC5-over-expressing human embryonic kidney 293 (HEK) cells and bovine aortic endothelial cells (BAECs) using fluorescent Ca(2+) imaging and electrophysiological techniques.
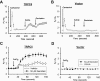
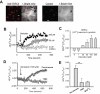
Key results: In TRPC5-over-expressing HEK cells, genistein stimulated TRPC5-mediated Ca(2+) influx, concentration dependently (EC(50)= 93 microM). Genistein and lanthanum activated TRPC5 channels synergistically. Effects of genistein on TRPC5 channels were mimicked by daidzein (100 microM), a genistein analogue inactive as a tyrosine kinase inhibitor, but not by known tyrosine kinase inhibitors herbimycin (2 microM), PP2 (20 microM) and lavendustin A (10 microM). Action of genistein on TRPC5 channels was not affected by an oestrogen receptor inhibitor ICI-182780 (50 microM) or a phospholipase C inhibitor U73122 (10 microM), suggesting genistein did not act through oestrogen receptors or phospholipase C. In BAECs, genistein (100 microM) stimulated TRPC5-mediated Ca(2+) influx. In patch clamp studies, both genistein (50 microM) and daidzein (50 microM) augmented TRPC5-mediated whole-cell cation current in TRPC5 over-expressing HEK cells. Genistein stimulated TRPC5 channel activity in excised inside-out membrane patch, suggesting that its action was relatively direct and did not require cytosolic factors.
Conclusions and implications: The present study is the first to demonstrate stimulation of a TRP channel by isoflavones. Genistein is a lipophilic compound able to stimulate TRPC5 activity in TRPC5-over-expressing HEK cells and in native vascular endothelial cells.
Figures
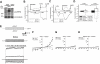



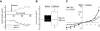
Similar articles
-
Inhibitory effects of genistein on ATP-sensitive K+ channels in rabbit portal vein smooth muscle.Br J Pharmacol. 1997 Dec;122(7):1395-404. doi: 10.1038/sj.bjp.0701532. Br J Pharmacol. 1997. PMID: 9421287 Free PMC article.
-
Ca2+-calmodulin-dependent myosin light chain kinase is essential for activation of TRPC5 channels expressed in HEK293 cells.J Physiol. 2006 Jan 15;570(Pt 2):219-35. doi: 10.1113/jphysiol.2005.097998. Epub 2005 Nov 10. J Physiol. 2006. PMID: 16284075 Free PMC article.
-
Influence of muscarinic agonists and tyrosine kinase inhibitors on L-type Ca(2+)Channels in human and bovine trabecular meshwork cells.Exp Eye Res. 2000 Mar;70(3):285-93. doi: 10.1006/exer.1999.0785. Exp Eye Res. 2000. PMID: 10712815
-
Inhibition by genistein of the hyperpolarization-activated cation current in porcine sino-atrial node cells.Br J Pharmacol. 1999 Nov;128(6):1284-90. doi: 10.1038/sj.bjp.0702903. Br J Pharmacol. 1999. PMID: 10578143 Free PMC article.
-
TRPC5 in cardiovascular diseases.Rev Cardiovasc Med. 2021 Mar 30;22(1):127-135. doi: 10.31083/j.rcm.2021.01.212. Rev Cardiovasc Med. 2021. PMID: 33792254 Review.
Cited by
-
Insulin increases surface expression of TRPC6 channels in podocytes: role of NADPH oxidases and reactive oxygen species.Am J Physiol Renal Physiol. 2012 Feb 1;302(3):F298-307. doi: 10.1152/ajprenal.00423.2011. Epub 2011 Oct 26. Am J Physiol Renal Physiol. 2012. PMID: 22031853 Free PMC article.
-
Aortic Baroreceptors Display Higher Mechanosensitivity than Carotid Baroreceptors.Front Physiol. 2016 Aug 31;7:384. doi: 10.3389/fphys.2016.00384. eCollection 2016. Front Physiol. 2016. PMID: 27630578 Free PMC article.
-
In pursuit of small molecule chemistry for calcium-permeable non-selective TRPC channels -- mirage or pot of gold?Br J Pharmacol. 2013 Oct;170(3):459-74. doi: 10.1111/bph.12274. Br J Pharmacol. 2013. PMID: 23763262 Free PMC article. Review.
-
Nitric oxide lacks direct effect on TRPC5 channels but suppresses endogenous TRPC5-containing channels in endothelial cells.Pflugers Arch. 2010 Jun;460(1):121-30. doi: 10.1007/s00424-010-0823-3. Pflugers Arch. 2010. PMID: 20390293 Free PMC article.
-
Plasma membrane mechanical stress activates TRPC5 channels.PLoS One. 2015 Apr 7;10(4):e0122227. doi: 10.1371/journal.pone.0122227. eCollection 2015. PLoS One. 2015. PMID: 25849346 Free PMC article.
References
-
- Akiyama T, Ishida J, Nakagawa S, Ogawara H, Watanabe S, Itoh N, et al. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J Biol Chem. 1987;262:5592–5595. - PubMed
-
- Arora A, Byrem TM, Nair MG, Strasburg GM. Modulation of liposomal membrane fluidity by flavonoids and isoflavonoids. Arch Biochem Biophys. 2000;373:102–109. - PubMed
-
- Barnes S, Boersma B, Patel R, Kirk M, Darley-Usmar VM, Kim H, et al. Isoflavonoids and chronic disease: mechanisms of action. Biofactors. 2000;12:209–215. - PubMed
-
- Beech DJ. Bipolar phospholipid sensing by TRPC5 calcium channel. Biochem Soc Trans. 2007a;35(Pt 1):101–104. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

