A common intracellular allosteric binding site for antagonists of the CXCR2 receptor
- PMID: 20233217
- PMCID: PMC2850400
- DOI: 10.1111/j.1476-5381.2009.00623.x
A common intracellular allosteric binding site for antagonists of the CXCR2 receptor
Abstract
Background and purpose: We have previously shown that SB265610 (1-(2-bromo-phenyl)-3-(7-cyano-3H-benzotriazol-4-yl)-urea) behaves as an allosteric, inverse agonist at the C-X-C chemokine (CXCR)2 receptor. The aim of this study was to determine whether SB265610, in addition to two other known antagonists, bind to either of the two putative, topographically distinct, allosteric binding sites previously reported in the Literature.
Experimental approach: Ten single point mutations were introduced into the CXCR2 receptor using site-directed mutagenesis. Three CXCR2 antagonists were investigated, SB265610, Pteridone-1 (2-(2,3 difluoro-benzylsulphanyl)-4-((R)-2-hydroxy-1-methyl-ethylamino)-8H-pteridin-7-one) and Sch527123 (2-hydroxy-N,N-dimethyl-3-{2-[[(R)-1-(5-methyl-furan-2-yl)-propyl]amino]-3,4-dioxo-cyclobut-1enylamino}-benzamide), and the effect of these mutations on their binding affinity and ability to inhibit interleukin-8-stimulated binding of [(35)S]GTPgammaS was examined.
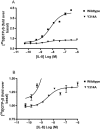
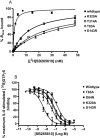
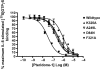
Key results: Seven of the nine mutations introduced into the C-terminal domain and intracellular loops of the receptor produced a significant reduction in affinity at least one of the antagonists tested. Of those seven mutations, three produced a significant reduction in the affinity of all three antagonists, namely K320A, Y314A and D84N. In all but one mutation, the changes observed on antagonist affinity were matched with effects on inhibition of interleukin-8-stimulated [(35)S]GTPgammaS binding.
Conclusions and implications: These antagonists bind to a common intracellular, allosteric, binding site of the CXCR2 receptor, which has been further delineated. As many of these mutations are close to the site of G protein coupling or to a region of the receptor that is responsible for the transduction of the activation signal, our results suggest a molecular mechanism for the inhibition of receptor activation.
Figures
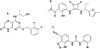
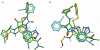
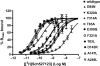

Similar articles
-
SB265610 is an allosteric, inverse agonist at the human CXCR2 receptor.Br J Pharmacol. 2009 Sep;158(1):328-38. doi: 10.1111/j.1476-5381.2009.00182.x. Epub 2009 Apr 24. Br J Pharmacol. 2009. PMID: 19422399 Free PMC article.
-
Combined anti CXC receptors 1 and 2 therapy is a promising anti-inflammatory treatment for respiratory diseases by reducing neutrophil migration and activation.Pulm Pharmacol Ther. 2015 Oct;34:37-45. doi: 10.1016/j.pupt.2015.08.002. Epub 2015 Aug 10. Pulm Pharmacol Ther. 2015. PMID: 26271598 Review.
-
Evaluation of potent and selective small-molecule antagonists for the CXCR2 chemokine receptor.J Med Chem. 2004 Mar 11;47(6):1319-21. doi: 10.1021/jm034248l. J Med Chem. 2004. PMID: 14998320
-
Nonpeptidergic allosteric antagonists differentially bind to the CXCR2 chemokine receptor.J Pharmacol Exp Ther. 2009 May;329(2):783-90. doi: 10.1124/jpet.108.148387. Epub 2009 Feb 3. J Pharmacol Exp Ther. 2009. PMID: 19190236
-
Discovery of 3,4-diaminocyclobut-3-ene-1,2-dione-based CXCR2 receptor antagonists for the treatment of inflammatory disorders.Curr Top Med Chem. 2010;10(13):1339-50. doi: 10.2174/156802610791561246. Curr Top Med Chem. 2010. PMID: 20536426 Review.
Cited by
-
What Do Structures Tell Us About Chemokine Receptor Function and Antagonism?Annu Rev Biophys. 2017 May 22;46:175-198. doi: 10.1146/annurev-biophys-051013-022942. Annu Rev Biophys. 2017. PMID: 28532213 Free PMC article. Review.
-
Identification of an antithrombotic allosteric modulator that acts through helix 8 of PAR1.Proc Natl Acad Sci U S A. 2011 Feb 15;108(7):2951-6. doi: 10.1073/pnas.1014863108. Epub 2011 Jan 31. Proc Natl Acad Sci U S A. 2011. PMID: 21282664 Free PMC article.
-
Parmodulins inhibit thrombus formation without inducing endothelial injury caused by vorapaxar.Blood. 2015 Mar 19;125(12):1976-85. doi: 10.1182/blood-2014-09-599910. Epub 2015 Jan 13. Blood. 2015. PMID: 25587041 Free PMC article.
-
Emerging concepts and approaches for chemokine-receptor drug discovery.Expert Opin Drug Discov. 2010 Nov;5(11):1109-22. doi: 10.1517/17460441.2010.525633. Expert Opin Drug Discov. 2010. PMID: 21132095 Free PMC article. Review.
-
International Union of Basic and Clinical Pharmacology. [corrected]. LXXXIX. Update on the extended family of chemokine receptors and introducing a new nomenclature for atypical chemokine receptors.Pharmacol Rev. 2013 Nov 11;66(1):1-79. doi: 10.1124/pr.113.007724. Print 2014. Pharmacol Rev. 2013. PMID: 24218476 Free PMC article. Review.
References
-
- Allegretti M, Bertini R, Bizzarri C, Beccari A, Mantovani A, Locati M. Allosteric inhibitors of chemoattractant receptors: opportunities and pitfalls. Trends Pharmacol Sci. 2008;29(6):280–286. - PubMed
-
- Andrews G, Jones C, Wreggett KA. An intracellular allosteric site for a specific class of antagonists of the CC chemokine G protein-coupled receptors CCR4 and CCR5. Mol Pharmacol. 2008;73(3):855–867. - PubMed
-
- Artursson P, Tavelin S. Caco-2 and emerging alternatives for prediction of intestinal drug transport: a general overview. In: van de Waterbeemd H, Lennernäs H, Artursson P, editors. Methods and Principles in Medicinal Chemistry. München, Germany: John Wiley & Sons; 2003. pp. 72–82.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

