Effect of dexmedetomidine versus lorazepam on outcome in patients with sepsis: an a priori-designed analysis of the MENDS randomized controlled trial
- PMID: 20233428
- PMCID: PMC2887145
- DOI: 10.1186/cc8916
Effect of dexmedetomidine versus lorazepam on outcome in patients with sepsis: an a priori-designed analysis of the MENDS randomized controlled trial
Erratum in
- Crit Care. 2011;15(1):402
Abstract
Introduction: Benzodiazepines and alpha2 adrenoceptor agonists exert opposing effects on innate immunity and mortality in animal models of infection. We hypothesized that sedation with dexmedetomidine (an alpha2 adrenoceptor agonist), as compared with lorazepam (a benzodiazepine), would provide greater improvements in clinical outcomes among septic patients than among non-septic patients.
Methods: In this a priori-determined subgroup analysis of septic vs non-septic patients from the MENDS double-blind randomized controlled trial, adult medical/surgical mechanically ventilated patients were randomized to receive dexmedetomidine-based or lorazepam-based sedation for up to 5 days. Delirium and other clinical outcomes were analyzed comparing sedation groups, adjusting for clinically relevant covariates as well as assessing interactions between sedation group and sepsis.
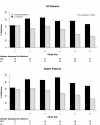
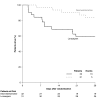
Results: Of the 103 patients randomized, 63 (31 dexmedetomidine; 32 lorazepam) were admitted with sepsis and 40 (21 dexmedetomidine; 19 lorazepam) without sepsis. Baseline characteristics were similar between treatment groups for both septic and non-septic patients. Compared with septic patients who received lorazepam, the dexmedetomidine septic patients had 3.2 more delirium/coma-free days (DCFD) on average (95% CI for difference, 1.1 to 4.9), 1.5 (-0.1, 2.8) more delirium-free days (DFD) and 6 (0.3, 11.1) more ventilator-free days (VFD). The beneficial effects of dexmedetomidine were more pronounced in septic patients than in non-septic patients for both DCFDs and VFDs (P-value for interaction = 0.09 and 0.02 respectively). Additionally, sedation with dexmedetomidine, compared with lorazepam, reduced the daily risk of delirium [OR, CI 0.3 (0.1, 0.7)] in both septic and non-septic patients (P-value for interaction = 0.94). Risk of dying at 28 days was reduced by 70% [hazard ratio 0.3 (0.1, 0.9)] in dexmedetomidine patients with sepsis as compared to the lorazepam patients; this reduction in death was not seen in non-septic patients (P-value for interaction = 0.11).
Conclusions: In this subgroup analysis, septic patients receiving dexmedetomidine had more days free of brain dysfunction and mechanical ventilation and were less likely to die than those that received a lorazepam-based sedation regimen. These results were more pronounced in septic patients than in non-septic patients. Prospective clinical studies and further preclinical mechanistic studies are needed to confirm these results.
Trial registration: NCT00095251.
Figures

Comment in
-
The complex interplay between delirium, sepsis and sedation.Crit Care. 2010;14(3):164. doi: 10.1186/cc9038. Epub 2010 Jun 14. Crit Care. 2010. PMID: 20587084 Free PMC article.
-
Alpha-2 adrenoceptor agonists and sepsis: improved survival?Crit Care. 2010;14(4):429; author reply 429. doi: 10.1186/cc9096. Epub 2010 Jul 29. Crit Care. 2010. PMID: 20727233 Free PMC article. No abstract available.
Similar articles
-
The complex interplay between delirium, sepsis and sedation.Crit Care. 2010;14(3):164. doi: 10.1186/cc9038. Epub 2010 Jun 14. Crit Care. 2010. PMID: 20587084 Free PMC article.
-
Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: the MENDS randomized controlled trial.JAMA. 2007 Dec 12;298(22):2644-53. doi: 10.1001/jama.298.22.2644. JAMA. 2007. PMID: 18073360 Clinical Trial.
-
Effect of Dexmedetomidine on Mortality and Ventilator-Free Days in Patients Requiring Mechanical Ventilation With Sepsis: A Randomized Clinical Trial.JAMA. 2017 Apr 4;317(13):1321-1328. doi: 10.1001/jama.2017.2088. JAMA. 2017. PMID: 28322414 Free PMC article. Clinical Trial.
-
Dexmedetomidine: A Review of Its Use for Sedation in the Intensive Care Setting.Drugs. 2015 Jul;75(10):1119-30. doi: 10.1007/s40265-015-0419-5. Drugs. 2015. PMID: 26063213 Review.
-
Effect of Dexmedetomidine on duration of mechanical ventilation in septic patients: a systematic review and meta-analysis.BMC Pulm Med. 2020 Feb 17;20(1):42. doi: 10.1186/s12890-020-1065-6. BMC Pulm Med. 2020. PMID: 32066417 Free PMC article.
Cited by
-
Dexmedetomidine versus standard therapy with fentanyl for sedation in mechanically ventilated premature neonates.J Pediatr Pharmacol Ther. 2012 Jul;17(3):252-62. doi: 10.5863/1551-6776-17.3.252. J Pediatr Pharmacol Ther. 2012. PMID: 23258968 Free PMC article.
-
Circulatory effects of dexmedetomidine in early sepsis: a randomised controlled experimental study.Naunyn Schmiedebergs Arch Pharmacol. 2020 Jan;393(1):89-97. doi: 10.1007/s00210-019-01713-3. Epub 2019 Aug 17. Naunyn Schmiedebergs Arch Pharmacol. 2020. PMID: 31422445
-
Sepsis-associated encephalopathy.CMAJ. 2018 Sep 10;190(36):E1083. doi: 10.1503/cmaj.180454. CMAJ. 2018. PMID: 30201616 Free PMC article. No abstract available.
-
How should dexmedetomidine and clonidine be prescribed in the critical care setting?Rev Bras Ter Intensiva. 2021 Oct-Dec;33(4):600-615. doi: 10.5935/0103-507X.20210087. Epub 2022 Jan 24. Rev Bras Ter Intensiva. 2021. PMID: 35081245 Free PMC article.
-
Dexmedetomidine: present and future directions.Korean J Anesthesiol. 2019 Aug;72(4):323-330. doi: 10.4097/kja.19259. Epub 2019 Jun 21. Korean J Anesthesiol. 2019. PMID: 31220910 Free PMC article. Review.
References
-
- Ely EW, Margolin R, Francis J, May L, Truman B, Dittus R, Speroff T, Gautam S, Bernard GR, Inouye SK. Evaluation of delirium in critically ill patients: validation of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) Crit Care Med. 2001;29:1370–1379. doi: 10.1097/00003246-200107000-00012. - DOI - PubMed

