A promising approach for treatment of tumor-induced bone diseases: utilizing bisphosphonate derivatives of nucleoside antimetabolites
- PMID: 20233612
- PMCID: PMC2892200
- DOI: 10.1016/j.bone.2010.03.006
A promising approach for treatment of tumor-induced bone diseases: utilizing bisphosphonate derivatives of nucleoside antimetabolites
Abstract
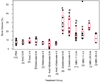
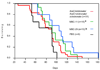
Despite palliative treatments, tumor-induced bone disease (TIBD) remains highly debilitating for many cancer patients and progression typically results in death within two years. Therefore, more effective therapies with enhanced anti-resorptive and cytotoxic characteristics are needed. We developed bisphosphonate-chemotherapeutic conjugates designed to bind bone and hydrolyze, releasing both compounds, thereby targeting both osteoclasts and tumor cells. This study examined the effects of our lead compound, MBC-11 (the anhydride formed between arabinocytidine (AraC)-5'-phosphate and etidronate), on bone tumor burden, bone volume, femur bone mineral density (BMD), and overall survival using two distinct mouse models of TIBD, the 4T1/luc breast cancer and the KAS-6/1-MIP1alpha multiple myeloma models. In mice orthotopically inoculated with 4T1/luc mouse mammary cells, MBC-11 (0.04 microg/day; s.c.) reduced the incidence of bone metastases to 40% (4/10), compared to 90% (9/10; p=0.057) and 100% (5/5; p=0.04) of PBS- or similarly-dosed, zoledronate-treated mice, respectively. MBC-11 also significantly decreased bone tumor burden compared to PBS- or zoledronate-treated mice (p=0.021, p=0.017, respectively). MBC-11 and zoledronate (0.04 microg/day) significantly increased bone volume by two- and four-fold, respectively, compared to PBS-treated mice (p=0.005, p<0.001, respectively). In mice systemically injected with human multiple myeloma KAS-6/1-MIP1alpha cells, 0.04 and 4.0 microg/day MBC-11 improved femur BMD by 13% and 16%, respectively, compared to PBS (p=0.025, p=0.017, respectively) at 10 weeks post-tumor cell injection and increased mean survival to 95 days compared to 77 days in mice treated with PBS (p=0.047). Similar doses of zoledronate also improved femur BMD (p< or =0.01 vs PBS) and increased mean survival to 86 days, but this was not significantly different than in PBS-treated mice (p=0.53). These results demonstrate that MBC-11 decreases bone tumor burden, maintains bone structure, and may increase overall survival, warranting further investigation as a treatment for TIBD.
2010 Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures










Similar articles
-
NOD/SCID-GAMMA mice are an ideal strain to assess the efficacy of therapeutic agents used in the treatment of myeloma bone disease.PLoS One. 2015 Mar 13;10(3):e0119546. doi: 10.1371/journal.pone.0119546. eCollection 2015. PLoS One. 2015. PMID: 25768011 Free PMC article.
-
Ibandronate decreases bone disease development and osteoclast stimulatory activity in an in vivo model of human myeloma.Exp Hematol. 2001 Apr;29(4):441-7. doi: 10.1016/s0301-472x(01)00618-x. Exp Hematol. 2001. PMID: 11301184
-
Combined treatment with a transforming growth factor beta inhibitor (1D11) and bortezomib improves bone architecture in a mouse model of myeloma-induced bone disease.Bone. 2016 Oct;91:81-91. doi: 10.1016/j.bone.2016.07.007. Epub 2016 Jul 14. Bone. 2016. PMID: 27423464 Free PMC article.
-
Bisphosphonate therapy in the treatment of multiple myeloma.Curr Pharm Des. 2010;16(27):3028-36. doi: 10.2174/138161210793563608. Curr Pharm Des. 2010. PMID: 20722619 Review.
-
Therapy insight: the risks and benefits of bisphosphonates for the treatment of tumor-induced bone disease.Nat Clin Pract Oncol. 2007 Jan;4(1):42-55. doi: 10.1038/ncponc0688. Nat Clin Pract Oncol. 2007. PMID: 17183355 Review.
Cited by
-
Bisphosphonate conjugation for bone specific drug targeting.Bone Rep. 2018 Jul 3;9:47-60. doi: 10.1016/j.bonr.2018.06.007. eCollection 2018 Dec. Bone Rep. 2018. PMID: 29992180 Free PMC article. Review.
-
Engineering small-molecule and protein drugs for targeting bone tumors.Mol Ther. 2024 May 1;32(5):1219-1237. doi: 10.1016/j.ymthe.2024.03.001. Epub 2024 Mar 6. Mol Ther. 2024. PMID: 38449313 Free PMC article. Review.
-
Drug delivery nanocarriers and recent advances ventured to improve therapeutic efficacy against osteosarcoma: an overview.J Egypt Natl Canc Inst. 2021 Feb 8;33(1):4. doi: 10.1186/s43046-021-00059-3. J Egypt Natl Canc Inst. 2021. PMID: 33555490 Review.
-
Bisphosphonates for delivering drugs to bone.Br J Pharmacol. 2021 May;178(9):2008-2025. doi: 10.1111/bph.15251. Epub 2021 Apr 10. Br J Pharmacol. 2021. PMID: 32876338 Free PMC article. Review.
-
Gemcitabine-Ibandronate Conjugate Enables the Bone-Targeted Combination Therapy in Bone Cancer: Synthesis and Efficacy in Combination with Docetaxel.Bioconjug Chem. 2021 Dec 15;32(12):2530-2539. doi: 10.1021/acs.bioconjchem.1c00507. Epub 2021 Nov 15. Bioconjug Chem. 2021. PMID: 34779607 Free PMC article.
References
-
- Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006;12(20 Pt 2):6243s–6249s. - PubMed
-
- Lipton A, Berenson JR, Body JJ, Boyce BF, Bruland OS, Carducci MA, Cleeland CS, Clohisy DR, Coleman RE, Cook RJ, Guise TA, Pearse RN, Powles TJ, Rogers MJ, Roodman GD, Smith MR, Suva LJ, Vessella RL, Weilbaecher KN, King L. Advances in treating metastatic bone cancer: summary statement for the First Cambridge Conference. Clin Cancer Res. 2006;12(20 Pt 2):6209s–6212s. - PMC - PubMed
-
- Aapro M, Abrahamsson PA, Body JJ, Coleman RE, Colomer R, Costa L, Crino L, Dirix L, Gnant M, Gralow J, Hadji P, Hortobagyi GN, Jonat W, Lipton A, Monnier A, Paterson AH, Rizzoli R, Saad F, Thurlimann B. Guidance on the use of bisphosphonates in solid tumours: recommendations of an international expert panel. Ann Oncol. 2008;19(3):420–432. - PubMed
-
- Body J-J. Bisphosphonates for malignancy-related bone disease: current status, future developments. Supportive Care in Cancer. 2006;V14(5):408–418. - PubMed
-
- Costa L. Bisphosphonates: reducing the risk of skeletal complications from bone metastasis. Breast. 2007;16 Suppl 3:S16–S20. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

