Integration of general amino acid control and target of rapamycin (TOR) regulatory pathways in nitrogen assimilation in yeast
- PMID: 20233714
- PMCID: PMC2878067
- DOI: 10.1074/jbc.M110.121947
Integration of general amino acid control and target of rapamycin (TOR) regulatory pathways in nitrogen assimilation in yeast
Abstract
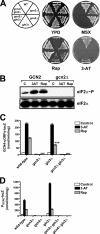
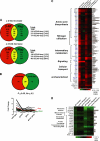
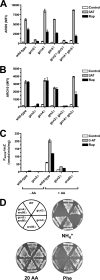
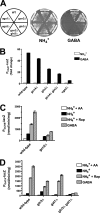
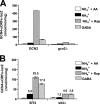
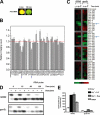
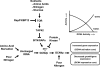
Two important nutrient-sensing and regulatory pathways, the general amino acid control (GAAC) and the target of rapamycin (TOR), participate in the control of yeast growth and metabolism during changes in nutrient availability. Amino acid starvation activates the GAAC through Gcn2p phosphorylation of translation factor eIF2 and preferential translation of GCN4, a transcription activator. TOR senses nitrogen availability and regulates transcription factors such as Gln3p. We used microarray analyses to address the integration of the GAAC and TOR pathways in directing the yeast transcriptome during amino acid starvation and rapamycin treatment. We found that GAAC is a major effector of the TOR pathway, with Gcn4p and Gln3p each inducing a similar number of genes during rapamycin treatment. Although Gcn4p activates a common core of 57 genes, the GAAC directs significant variations in the transcriptome during different stresses. In addition to inducing amino acid biosynthetic genes, Gcn4p in conjunction with Gln3p activates genes required for the assimilation of secondary nitrogen sources such as gamma-aminobutyric acid (GABA). Gcn2p activation upon shifting to secondary nitrogen sources is suggested to occur by means of a dual mechanism. First, Gcn2p is induced by the release of TOR repression through a mechanism involving Sit4p protein phosphatase. Second, this eIF2 kinase is activated by select uncharged tRNAs, which were shown to accumulate during the shift to the GABA medium. This study highlights the mechanisms by which the GAAC and TOR pathways are integrated to recognize changing nitrogen availability and direct the transcriptome for optimal growth adaptation.
Figures


Similar articles
-
Regulation of Sensing, Transportation, and Catabolism of Nitrogen Sources in Saccharomyces cerevisiae.Microbiol Mol Biol Rev. 2018 Feb 7;82(1):e00040-17. doi: 10.1128/MMBR.00040-17. Print 2018 Jun. Microbiol Mol Biol Rev. 2018. PMID: 29436478 Free PMC article. Review.
-
General Amino Acid Control and 14-3-3 Proteins Bmh1/2 Are Required for Nitrogen Catabolite Repression-Sensitive Regulation of Gln3 and Gat1 Localization.Genetics. 2017 Feb;205(2):633-655. doi: 10.1534/genetics.116.195800. Epub 2016 Dec 22. Genetics. 2017. PMID: 28007891 Free PMC article.
-
The yeast eIF4E-associated protein Eap1p attenuates GCN4 translation upon TOR-inactivation.FEBS Lett. 2005 Apr 25;579(11):2433-8. doi: 10.1016/j.febslet.2005.03.043. FEBS Lett. 2005. PMID: 15848184
-
Aminoacyl-tRNA quality control is required for efficient activation of the TOR pathway regulator Gln3p.RNA Biol. 2018;15(4-5):594-603. doi: 10.1080/15476286.2017.1379635. Epub 2017 Oct 6. RNA Biol. 2018. PMID: 28910581 Free PMC article. Review.
-
Yeast protein expression profile during acetic acid-induced apoptosis indicates causal involvement of the TOR pathway.Proteomics. 2009 Feb;9(3):720-32. doi: 10.1002/pmic.200700816. Proteomics. 2009. PMID: 19137548
Cited by
-
A network-based approach on elucidating the multi-faceted nature of chronological aging in S. cerevisiae.PLoS One. 2011;6(12):e29284. doi: 10.1371/journal.pone.0029284. Epub 2011 Dec 21. PLoS One. 2011. PMID: 22216232 Free PMC article.
-
Effects of abolishing Whi2 on the proteome and nitrogen catabolite repression-sensitive protein production.G3 (Bethesda). 2022 Mar 4;12(3):jkab432. doi: 10.1093/g3journal/jkab432. G3 (Bethesda). 2022. PMID: 35100365 Free PMC article.
-
A plant virus movement protein regulates the Gcn2p kinase in budding yeast.PLoS One. 2011;6(11):e27409. doi: 10.1371/journal.pone.0027409. Epub 2011 Nov 8. PLoS One. 2011. PMID: 22087310 Free PMC article.
-
Regulation of Sensing, Transportation, and Catabolism of Nitrogen Sources in Saccharomyces cerevisiae.Microbiol Mol Biol Rev. 2018 Feb 7;82(1):e00040-17. doi: 10.1128/MMBR.00040-17. Print 2018 Jun. Microbiol Mol Biol Rev. 2018. PMID: 29436478 Free PMC article. Review.
-
General Amino Acid Control and 14-3-3 Proteins Bmh1/2 Are Required for Nitrogen Catabolite Repression-Sensitive Regulation of Gln3 and Gat1 Localization.Genetics. 2017 Feb;205(2):633-655. doi: 10.1534/genetics.116.195800. Epub 2016 Dec 22. Genetics. 2017. PMID: 28007891 Free PMC article.
References
-
- Hinnebusch A. G. (1997) J. Biol. Chem. 272, 21661–21664 - PubMed
-
- Hinnebusch A. G. (2005) Annu. Rev. Microbiol. 59, 407–450 - PubMed
-
- Wek R. C., Staschke K. A., Narasimhan J. (2004) Topics in Current Genetics: Nutrient-induced Responses in Eukaryotic Cells (Winderickx J. G., Taylor P. M. eds) pp. 177–199, Springer, Berlin/Heidelberg
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

