Mammalian numb-interacting protein 1/dual oxidase maturation factor 1 directs neuronal fate in stem cells
- PMID: 20233719
- PMCID: PMC2878559
- DOI: 10.1074/jbc.M109.084616
Mammalian numb-interacting protein 1/dual oxidase maturation factor 1 directs neuronal fate in stem cells
Abstract
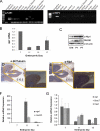
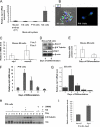
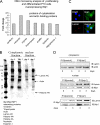
In this study, we describe a role for the mammalian Numb-interacting protein 1 (Nip1) in regulation of neuronal differentiation in stem cells. The expression of Nip1 was detected in the developing mouse brain, embryonic stem cells, primary neuronal stem cells, and retinoic acid-treated P19 embryonal carcinoma cells. The highest expression of Nip1 was observed in undifferentiated neuronal stem cells and was associated with Duox1-mediated reactive oxygen species ROS production. Ectopic nip1 expression in P19 embryonal carcinoma cells induced neuronal differentiation, and this phenotype was also linked to elevated ROS production. The neuronal differentiation in nip1-overexpressing P19 cells was achieved in a retinoic acid-independent manner and was corroborated by an increase in the expression of the neuronal basic helix-loop-helix transcription factors and neural-lineage cell markers. Furthermore, depletion of nip1 by short hairpin RNA led to a decrease in the expression of neuronal basic helix-loop-helix transcription factors and ROS. However, inhibition of ROS production in nip1-overexpressing P19 cells restricted but did not extinguish neuronal differentiation. Microarray and mass spectrometry analysis identified intermediate filaments as the principal cytoskeletal elements affected by up-regulation of nip1. We show here the first evidence for a functional interaction between Nip1 and a component of the nuclear lamina, lamin A/C. associated with a neuronal-specific phenotype. Taken together, our data reveal an important role for Nip1 in the guidance of neuronal differentiation through ROS generation and modulation of intermediate filaments and implicate Nip1 as a novel intrinsic regulator of neuronal cell fate.
Figures





Similar articles
-
NIP1/DUOXA1 expression in epithelial breast cancer cells: regulation of cell adhesion and actin dynamics.Breast Cancer Res Treat. 2010 Feb;119(3):773-86. doi: 10.1007/s10549-009-0372-7. Epub 2009 Mar 26. Breast Cancer Res Treat. 2010. PMID: 19322654
-
Regulated expression of Wnt family members during neuroectodermal differentiation of P19 embryonal carcinoma cells: overexpression of Wnt-1 perturbs normal differentiation-specific properties.Dev Biol. 1994 Nov;166(1):300-10. doi: 10.1006/dbio.1994.1316. Dev Biol. 1994. PMID: 7958454
-
Cdc42-mTOR signaling pathway controls Hes5 and Pax6 expression in retinoic acid-dependent neural differentiation.J Biol Chem. 2009 Feb 20;284(8):5107-18. doi: 10.1074/jbc.M807745200. Epub 2008 Dec 19. J Biol Chem. 2009. PMID: 19097998 Free PMC article.
-
Neurotransmitter receptor expression and activity during neuronal differentiation of embryonal carcinoma and stem cells: from basic research towards clinical applications.Cell Prolif. 2006 Aug;39(4):281-300. doi: 10.1111/j.1365-2184.2006.00385.x. Cell Prolif. 2006. PMID: 16872363 Free PMC article. Review.
-
[Expression and Regulation of Tal2 during Neuronal Differentiation in P19 Cells].Yakugaku Zasshi. 2017;137(1):61-71. doi: 10.1248/yakushi.16-00176. Yakugaku Zasshi. 2017. PMID: 28049897 Review. Japanese.
Cited by
-
Dual oxidase maturation factor 1 (DUOXA1) overexpression increases reactive oxygen species production and inhibits murine muscle satellite cell differentiation.Cell Commun Signal. 2014 Jan 11;12:5. doi: 10.1186/1478-811X-12-5. Cell Commun Signal. 2014. PMID: 24410844 Free PMC article.
-
Reactive oxygen species and the neuronal fate.Cell Mol Life Sci. 2012 Jan;69(2):215-21. doi: 10.1007/s00018-011-0807-2. Epub 2011 Sep 23. Cell Mol Life Sci. 2012. PMID: 21947442 Free PMC article. Review.
-
p53 in stem cells.World J Biol Chem. 2011 Sep 26;2(9):202-14. doi: 10.4331/wjbc.v2.i9.202. World J Biol Chem. 2011. PMID: 21949570 Free PMC article.
-
NFκB signaling regulates embryonic and adult neurogenesis.Front Biol (Beijing). 2012 Aug;7(4):10.1007/s11515-012-1233-z. doi: 10.1007/s11515-012-1233-z. Front Biol (Beijing). 2012. PMID: 24324484 Free PMC article.
-
ROS produced by NOX2 control in vitro development of cerebellar granule neurons development.ASN Neuro. 2015 Apr 13;7(2):1759091415578712. doi: 10.1177/1759091415578712. Print 2015 Mar-Apr. ASN Neuro. 2015. PMID: 25873309 Free PMC article.
References
-
- Johnson J. E., Birren S. J., Anderson D. J. (1990) Nature 346, 858–861 - PubMed
-
- Guillemot F., Lo L. C., Johnson J. E., Auerbach A., Anderson D. J., Joyner A. L. (1993) Cell 75, 463–476 - PubMed
-
- Akazawa C., Ishibashi M., Shimizu C., Nakanishi S., Kageyama R. (1995) J. Biol. Chem. 270, 8730–8738 - PubMed
-
- Ma Q., Kintner C., Anderson D. J. (1996) Cell 87, 43–52 - PubMed
-
- Sommer L., Ma Q., Anderson D. J. (1996) Mol. Cell. Neurosci. 8, 221–241 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

