Tipin-replication protein A interaction mediates Chk1 phosphorylation by ATR in response to genotoxic stress
- PMID: 20233725
- PMCID: PMC2878033
- DOI: 10.1074/jbc.M110.110304
Tipin-replication protein A interaction mediates Chk1 phosphorylation by ATR in response to genotoxic stress
Abstract
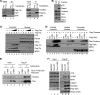
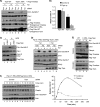
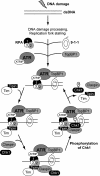
Mammalian Timeless is a multifunctional protein that performs essential roles in the circadian clock, chromosome cohesion, DNA replication fork protection, and DNA replication/DNA damage checkpoint pathways. The human Timeless exists in a tight complex with a smaller protein called Tipin (Timeless-interacting protein). Here we investigated the mechanism by which the Timeless-Tipin complex functions as a mediator in the ATR-Chk1 DNA damage checkpoint pathway. We find that the Timeless-Tipin complex specifically mediates Chk1 phosphorylation by ATR in response to DNA damage and replication stress through interaction of Tipin with the 34-kDa subunit of replication protein A (RPA). The Tipin-RPA interaction stabilizes Timeless-Tipin and Tipin-Claspin complexes on RPA-coated ssDNA and in doing so promotes Claspin-mediated phosphorylation of Chk1 by ATR. Our results therefore indicate that RPA-covered ssDNA not only supports recruitment and activation of ATR but also, through Tipin and Claspin, it plays an important role in the action of ATR on its critical downstream target Chk1.
Figures



Similar articles
-
Separation of intra-S checkpoint protein contributions to DNA replication fork protection and genomic stability in normal human fibroblasts.Cell Cycle. 2013 Jan 15;12(2):332-45. doi: 10.4161/cc.23177. Epub 2012 Jan 15. Cell Cycle. 2013. PMID: 23255133 Free PMC article.
-
Timeless functions independently of the Tim-Tipin complex to promote sister chromatid cohesion in normal human fibroblasts.Cell Cycle. 2011 May 15;10(10):1618-24. doi: 10.4161/cc.10.10.15613. Epub 2011 May 15. Cell Cycle. 2011. PMID: 21508667 Free PMC article.
-
Tim-Tipin dysfunction creates an indispensible reliance on the ATR-Chk1 pathway for continued DNA synthesis.J Cell Biol. 2009 Oct 5;187(1):15-23. doi: 10.1083/jcb.200905006. J Cell Biol. 2009. PMID: 19805627 Free PMC article.
-
Regulation of ATR-CHK1 signaling by ubiquitination of CLASPIN.Biochem Soc Trans. 2022 Oct 31;50(5):1471-1480. doi: 10.1042/BST20220729. Biochem Soc Trans. 2022. PMID: 36196914 Review.
-
Claspin - checkpoint adaptor and DNA replication factor.FEBS J. 2019 Feb;286(3):441-455. doi: 10.1111/febs.14594. Epub 2018 Jun 29. FEBS J. 2019. PMID: 29931808 Review.
Cited by
-
The Fork Protection Complex: A Regulatory Hub at the Head of the Replisome.Subcell Biochem. 2022;99:83-107. doi: 10.1007/978-3-031-00793-4_3. Subcell Biochem. 2022. PMID: 36151374
-
Human Tim-Tipin complex affects the biochemical properties of the replicative DNA helicase and DNA polymerases.Proc Natl Acad Sci U S A. 2013 Feb 12;110(7):2523-7. doi: 10.1073/pnas.1222494110. Epub 2013 Jan 28. Proc Natl Acad Sci U S A. 2013. PMID: 23359676 Free PMC article.
-
And-1 coordinates with Claspin for efficient Chk1 activation in response to replication stress.EMBO J. 2015 Aug 4;34(15):2096-110. doi: 10.15252/embj.201488016. Epub 2015 Jun 16. EMBO J. 2015. PMID: 26082189 Free PMC article.
-
The TIMELESS effort for timely DNA replication and protection.Cell Mol Life Sci. 2023 Mar 9;80(4):84. doi: 10.1007/s00018-023-04738-3. Cell Mol Life Sci. 2023. PMID: 36892674 Free PMC article. Review.
-
The Adaptive Mechanisms and Checkpoint Responses to a Stressed DNA Replication Fork.Int J Mol Sci. 2023 Jun 22;24(13):10488. doi: 10.3390/ijms241310488. Int J Mol Sci. 2023. PMID: 37445667 Free PMC article. Review.
References
-
- Sancar A., Lindsey-Boltz L. A., Unsal-Kaçmaz K., Linn S. (2004) Annu. Rev. Biochem. 73, 39–85 - PubMed
-
- Kerzendorfer C., O'Driscoll M. (2009) DNA Repair 8, 1139–1152 - PubMed
-
- Lee J. H., Paull T. T. (2007) Oncogene 26, 7741–7748 - PubMed
-
- Palm W., de Lange T. (2008) Annu. Rev. Genet. 42, 301–334 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

