Adjuvant chemotherapy use and adverse events among older patients with stage III colon cancer
- PMID: 20233821
- PMCID: PMC2893553
- DOI: 10.1001/jama.2010.272
Adjuvant chemotherapy use and adverse events among older patients with stage III colon cancer
Abstract
Context: Randomized trials suggest adjuvant chemotherapy is effective for older patients with stage III colon cancer. However, older patients are less likely to receive this therapy than younger patients, perhaps because of concern about adverse effects.
Objective: To evaluate adjuvant chemotherapy use and outcomes for older patients with stage III colon cancer from well-defined population-based settings and health care systems.
Design: Observational study of adjuvant chemotherapy use and outcomes by age using Poisson regression to estimate the number of adverse events adjusted for demographic and clinical factors, including comorbid illness and specific elements of chemotherapy regimens documented with clinically detailed medical record reviews and patient and surrogate surveys.
Setting: Five geographically defined regions (Alabama, Iowa, Los Angeles County, northern California, and North Carolina), 5 integrated health care delivery systems, and 15 Veterans Affairs hospitals.
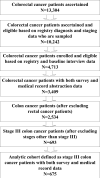
Patients: Six hundred seventy-five patients diagnosed with stage III colon cancer from 2003 through 2005 who underwent surgical resection and were followed up for as long as 15 months postdiagnosis.
Main outcome measures: Chemotherapy regimen, dose, duration, and annualized mean number of adverse events stratified by age.
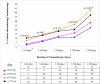
Results: Of 202 patients aged 75 years and older, 101 (50%) received adjuvant chemotherapy compared with 87% of 473 younger patients (difference, 37%; 95% confidence interval [CI], 30%-45%). Among patients who received adjuvant chemotherapy, 14 patients (14%) aged 75 years and older and 178 younger patients (44%) received a regimen containing oxaliplatin (difference, 30%; 95% CI, 21%-38%). Older patients were less likely to continue treatment, such that by 150 days, 99 patients (40%) aged 65 years and older and 68 younger patients (25%) had discontinued chemotherapy (difference, 15%; 95% CI, 7%-23%). Overall, 162 patients (24%) had at least 1 adverse clinical event, with more events among patients treated with vs without adjuvant chemotherapy (mean, 0.39 vs 0.16; difference, 0.23; 95% CI, 0.11-0.36; P < .001). Among patients receiving adjuvant chemotherapy, adjusted rates of late clinical adverse events were lower for patients 75 years and older (mean, 0.28) vs for younger patients (0.35 for ages 18-54 years, 0.52 for ages 55-64 years, and 0.45 for ages 65-74 years; P = .008 for any age effect).
Conclusion: Among patients with stage III colon cancer who underwent surgical resection and received adjuvant chemotherapy, older patients in the community received less-toxic and shorter chemotherapy regimens, and those treated had fewer adverse events than younger patients.
Figures
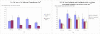
Comment in
-
Caring for patients with cancer.JAMA. 2010 Mar 17;303(11):1094-5. doi: 10.1001/jama.2010.285. JAMA. 2010. PMID: 20233832 No abstract available.
-
Adjuvant chemotherapy use among older patients with stage III colon cancer.JAMA. 2010 Jun 16;303(23):2353; author reply 2353-4. doi: 10.1001/jama.2010.775. JAMA. 2010. PMID: 20551402 No abstract available.
Similar articles
-
3-month versus 6-month adjuvant chemotherapy for patients with high-risk stage II and III colorectal cancer: 3-year follow-up of the SCOT non-inferiority RCT.Health Technol Assess. 2019 Dec;23(64):1-88. doi: 10.3310/hta23640. Health Technol Assess. 2019. PMID: 31852579 Free PMC article. Clinical Trial.
-
Assessment of Duration and Effects of 3 vs 6 Months of Adjuvant Chemotherapy in High-Risk Stage II Colorectal Cancer: A Subgroup Analysis of the TOSCA Randomized Clinical Trial.JAMA Oncol. 2020 Apr 1;6(4):547-551. doi: 10.1001/jamaoncol.2019.6486. JAMA Oncol. 2020. PMID: 32053133 Free PMC article. Clinical Trial.
-
Comparative effectiveness of oxaliplatin vs non-oxaliplatin-containing adjuvant chemotherapy for stage III colon cancer.J Natl Cancer Inst. 2012 Feb 8;104(3):211-27. doi: 10.1093/jnci/djr524. Epub 2012 Jan 20. J Natl Cancer Inst. 2012. PMID: 22266473 Free PMC article.
-
Safety and Efficacy of a Modified FLOX Adjuvant Regimen for Patients With Stage III Colorectal Cancer Treated in the Community.Clin Colorectal Cancer. 2017 Mar;16(1):65-72. doi: 10.1016/j.clcc.2016.07.001. Epub 2016 Jul 19. Clin Colorectal Cancer. 2017. PMID: 27515842
-
Improving outcomes for women aged 70 years or above with early breast cancer: research programme including a cluster RCT.Southampton (UK): National Institute for Health and Care Research; 2022 Jun. Southampton (UK): National Institute for Health and Care Research; 2022 Jun. PMID: 35793425 Free Books & Documents. Review.
Cited by
-
Oral Problems in Oncology Patients Undergoing Chemotherapy for Solid Tumors: A Prospective Observational Study.Cancers (Basel). 2023 Dec 29;16(1):176. doi: 10.3390/cancers16010176. Cancers (Basel). 2023. PMID: 38201603 Free PMC article.
-
Application of the Marginal Structural Model to Account for Suboptimal Adherence in a Randomized Controlled Trial.Contemp Clin Trials Commun. 2016 Dec 15;4:222-228. doi: 10.1016/j.conctc.2016.10.005. Epub 2016 Nov 3. Contemp Clin Trials Commun. 2016. PMID: 27900372 Free PMC article.
-
Targeting PLA2G16, a lipid metabolism gene, by Ginsenoside Compound K to suppress the malignant progression of colorectal cancer.J Adv Res. 2021 Jun 12;36:265-276. doi: 10.1016/j.jare.2021.06.009. eCollection 2022 Feb. J Adv Res. 2021. PMID: 35127176 Free PMC article.
-
Short- and long-term outcomes of laparoscopic surgery for colorectal cancer in the elderly aged over 80 years old versus non-elderly: a retrospective cohort study.BMC Geriatr. 2020 Nov 4;20(1):445. doi: 10.1186/s12877-020-01779-2. BMC Geriatr. 2020. PMID: 33148215 Free PMC article.
-
Real-world evidence on adjuvant chemotherapy in older adults with stage II/III colon cancer.World J Gastrointest Oncol. 2020 Jun 15;12(6):604-618. doi: 10.4251/wjgo.v12.i6.604. World J Gastrointest Oncol. 2020. PMID: 32699576 Free PMC article. Review.
References
-
- Moertel CG, Fleming TR, Macdonald JS, et al. Levamisole and fluorouracil for adjuvant therapy of resected colon carcinoma. N Engl J Med. 1990 Feb 8;322(6):352–358. - PubMed
-
- Laurie JA, Moertel CG, Fleming TR, et al. Surgical adjuvant therapy of large-bowel carcinoma: an evaluation of levamisole and the combination of levamisole and fluorouracil. The North Central Cancer Treatment Group and the Mayo Clinic. J Clin Oncol. 1989 Oct;7(10):1447–1456. - PubMed
-
- Zaniboni A, Labianca R, Marsoni S, et al. GIVIO-SITAC 01: A randomized trial of adjuvant 5-fluorouracil and folinic acid administered to patients with colon carcinoma--long term results and evaluation of the indicators of health-related quality of life. Gruppo Italiano Valutazione Interventi in Oncologia. Studio Italiano Terapia Adiuvante Colon. Cancer. 1998 Jun 1;82(11):2135–2144. - PubMed
-
- Andre T, Boni C, Mounedji-Boudiaf L, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004 Jun 3;350(23):2343–2351. - PubMed
-
- Sargent DJ, Goldberg RM, Jacobson SD, et al. A pooled analysis of adjuvant chemotherapy for resected colon cancer in elderly patients. N Engl J Med. 2001 Oct 11;345(15):1091–1097. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

