Multiscale model for the assessment of autonomic dysfunction in human endotoxemia
- PMID: 20233835
- PMCID: PMC2888557
- DOI: 10.1152/physiolgenomics.00184.2009
Multiscale model for the assessment of autonomic dysfunction in human endotoxemia
Abstract
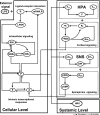
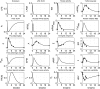
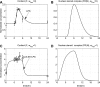
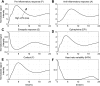
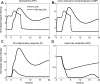
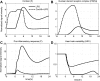
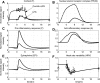
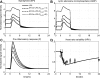
Severe injury and infection are associated with autonomic dysfunction. The realization that a dysregulation in autonomic function may predispose a host to excessive inflammatory processes has renewed interest in understanding the role of central nervous system (CNS) in modulating systemic inflammatory processes. Assessment of heart rate variability (HRV) has been used to evaluate systemic abnormalities and as a predictor of the severity of illness. Dissecting the relevance of neuroimmunomodulation in controlling inflammatory processes requires an understanding of the multiscale interplay between CNS and the immune response. A vital enabler in that respect is the development of a systems-based approach that integrates data across multiple scales, and models the emerging host response as the outcome of interactions of critical modules. Thus, a multiscale model of human endotoxemia, as a prototype model of systemic inflammation in humans, is proposed that integrates processes across the host from the cellular to the systemic host response level. At the cellular level interacting components are associated with elementary signaling pathways that propagate extracellular signals to the transcriptional response level. Further, essential modules associated with the neuroendocrine immune crosstalk are considered. Finally, at the systemic level, phenotypic expressions such as HRV are incorporated to assess systemic decomplexification indicative of the severity of the host response. Thus, the proposed work intends to associate acquired endocrine dysfunction with diminished HRV as a critical enabler for clarifying how cellular inflammatory processes and neural-based pathways mediate the links between patterns of autonomic control (HRV) and clinical outcomes.
Figures
Similar articles
-
A physiological model for autonomic heart rate regulation in human endotoxemia.Shock. 2011 Mar;35(3):229-39. doi: 10.1097/SHK.0b013e318200032b. Shock. 2011. PMID: 21063241 Free PMC article.
-
Low-dose steroid alters in vivo endotoxin-induced systemic inflammation but does not influence autonomic dysfunction.J Endotoxin Res. 2007;13(6):358-68. doi: 10.1177/0968051907086465. J Endotoxin Res. 2007. PMID: 18182463 Clinical Trial.
-
On heart rate variability and autonomic activity in homeostasis and in systemic inflammation.Math Biosci. 2014 Jun;252:36-44. doi: 10.1016/j.mbs.2014.03.010. Epub 2014 Mar 26. Math Biosci. 2014. PMID: 24680646 Free PMC article.
-
Translational applications of evaluating physiologic variability in human endotoxemia.J Clin Monit Comput. 2013 Aug;27(4):405-15. doi: 10.1007/s10877-012-9418-1. Epub 2012 Dec 1. J Clin Monit Comput. 2013. PMID: 23203205 Free PMC article. Review.
-
Heart rate variability in critical care medicine.Curr Opin Crit Care. 2002 Oct;8(5):371-5. doi: 10.1097/00075198-200210000-00002. Curr Opin Crit Care. 2002. PMID: 12357103 Review.
Cited by
-
Modeling autonomic regulation of cardiac function and heart rate variability in human endotoxemia.Physiol Genomics. 2011 Aug 24;43(16):951-64. doi: 10.1152/physiolgenomics.00040.2011. Epub 2011 Jun 14. Physiol Genomics. 2011. PMID: 21673075 Free PMC article.
-
Computational translation of genomic responses from experimental model systems to humans.PLoS Comput Biol. 2019 Jan 10;15(1):e1006286. doi: 10.1371/journal.pcbi.1006286. eCollection 2019 Jan. PLoS Comput Biol. 2019. PMID: 30629591 Free PMC article.
-
A physiological model for autonomic heart rate regulation in human endotoxemia.Shock. 2011 Mar;35(3):229-39. doi: 10.1097/SHK.0b013e318200032b. Shock. 2011. PMID: 21063241 Free PMC article.
-
The role of the hypothalamic-pituitary-adrenal axis in modulating seasonal changes in immunity.Physiol Genomics. 2016 Oct 1;48(10):719-738. doi: 10.1152/physiolgenomics.00006.2016. Epub 2016 Jun 24. Physiol Genomics. 2016. PMID: 27341833 Free PMC article.
-
Modeling physiologic variability in human endotoxemia.Crit Rev Biomed Eng. 2012;40(4):313-22. doi: 10.1615/critrevbiomedeng.v40.i4.60. Crit Rev Biomed Eng. 2012. PMID: 23140122 Free PMC article.
References
-
- Aldridge BB, Burke JM, Lauffenburger DA, Sorger PK. Physicochemical modelling of cell signalling pathways. Nat Cell Biol 8: 1195–1203, 2006 - PubMed
-
- Alvarez SM, Katsamanis Karavidas M, Coyle SM, Lu SE, Macor M, Oikawa LO, Lehrer PM, Calvano SE, Lowry SF. Low-dose steroid alters in vivo endotoxin-induced systemic inflammation but does not influence autonomic dysfunction. J Endotoxin Res 13: 358–368, 2007. - PubMed
-
- Andreasen AS, Krabbe KS, Krogh-Madsen R, Taudorf S, Pedersen BK, Moller K. Human endotoxemia as a model of systemic inflammation. Curr Med Chem 15: 1697–1705, 2008 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

