Ephrin-B2 regulates endothelial cell morphology and motility independently of Eph-receptor binding
- PMID: 20233847
- PMCID: PMC2848112
- DOI: 10.1242/jcs.061903
Ephrin-B2 regulates endothelial cell morphology and motility independently of Eph-receptor binding
Abstract
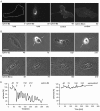
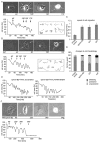
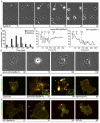
The transmembrane protein ephrin-B2 regulates angiogenesis, i.e. the formation of new blood vessels through endothelial sprouting, proliferation and remodeling processes. In addition to essential roles in the embryonic vasculature, ephrin-B2 expression is upregulated in the adult at sites of neovascularization, such as tumors and wounds. Ephrins are known to bind Eph receptor family tyrosine kinases on neighboring cells and trigger bidirectional signal transduction downstream of both interacting molecules. Here we show that ephrin-B2 dynamically modulates the motility and cellular morphology of isolated endothelial cells. Even in the absence of Eph-receptor binding, ephrin-B2 stimulates repeated cycling between actomyosin-dependent cell contraction and spreading episodes, which requires the presence of the C-terminal PDZ motif. Our results show that ephrin-B2 is a potent regulator of endothelial cell behavior, and indicate that the control of cell migration and angiogenesis by ephrins might involve both receptor-dependent and receptor-independent activities.
Figures




Similar articles
-
Ephrin-B2 controls VEGF-induced angiogenesis and lymphangiogenesis.Nature. 2010 May 27;465(7297):483-6. doi: 10.1038/nature09002. Nature. 2010. PMID: 20445537
-
Regulation of angiogenesis by Eph-ephrin interactions.Trends Cardiovasc Med. 2007 Jul;17(5):145-51. doi: 10.1016/j.tcm.2007.03.003. Trends Cardiovasc Med. 2007. PMID: 17574121 Review.
-
Regulation of signaling interactions and receptor endocytosis in growing blood vessels.Cell Adh Migr. 2014;8(4):366-77. doi: 10.4161/19336918.2014.970010. Cell Adh Migr. 2014. PMID: 25482636 Free PMC article. Review.
-
Ephrin-B2 regulates VEGFR2 function in developmental and tumour angiogenesis.Nature. 2010 May 27;465(7297):487-91. doi: 10.1038/nature08995. Epub 2010 May 5. Nature. 2010. PMID: 20445540
-
Rac-dependent trans-endocytosis of ephrinBs regulates Eph-ephrin contact repulsion.Nat Cell Biol. 2003 Oct;5(10):879-88. doi: 10.1038/ncb1044. Epub 2003 Sep 14. Nat Cell Biol. 2003. PMID: 12973357
Cited by
-
Ephrins and Eph Receptor Signaling in Tissue Repair and Fibrosis.Curr Rheumatol Rep. 2019 Apr 12;21(6):23. doi: 10.1007/s11926-019-0825-x. Curr Rheumatol Rep. 2019. PMID: 30980212 Free PMC article. Review.
-
Ephrin-Bs Drive Junctional Downregulation and Actin Stress Fiber Disassembly to Enable Wound Re-epithelialization.Cell Rep. 2015 Nov 17;13(7):1380-1395. doi: 10.1016/j.celrep.2015.09.085. Epub 2015 Nov 5. Cell Rep. 2015. PMID: 26549443 Free PMC article.
-
Ephrin-B2 reverse signaling regulates progression and lymph node metastasis of oral squamous cell carcinoma.PLoS One. 2017 Nov 30;12(11):e0188965. doi: 10.1371/journal.pone.0188965. eCollection 2017. PLoS One. 2017. PMID: 29190834 Free PMC article.
-
Identification of Eph receptor signaling as a regulator of autophagy and a therapeutic target in colorectal carcinoma.Mol Oncol. 2019 Nov;13(11):2441-2459. doi: 10.1002/1878-0261.12576. Epub 2019 Oct 23. Mol Oncol. 2019. PMID: 31545551 Free PMC article.
-
Behavioral improvement and regulation of molecules related to neuroplasticity in ischemic rat spinal cord treated with PEDF.Neural Plast. 2014;2014:451639. doi: 10.1155/2014/451639. Epub 2014 Jul 3. Neural Plast. 2014. PMID: 25110592 Free PMC article.
References
-
- Adams R. H., Wilkinson G. A., Weiss C., Diella F., Gale N. W., Deutsch U., Risau W., Klein R. (1999). Roles of ephrinB ligands and EphB receptors in cardiovascular development: demarcation of arterial/venous domains, vascular morphogenesis, and sprouting angiogenesis. Genes Dev. 13, 295-306 - PMC - PubMed
-
- Brantley-Sieders D. M., Chen J. (2004). Eph receptor tyrosine kinases in angiogenesis: from development to disease. Angiogenesis 7, 17-28 - PubMed
-
- Bruckner K., Pablo Labrador J., Scheiffele P., Herb A., Seeburg P. H., Klein R. (1999). EphrinB ligands recruit GRIP family PDZ adaptor proteins into raft membrane microdomains. Neuron 22, 511-524 - PubMed
-
- Carvalho R. F., Beutler M., Marler K. J., Knoll B., Becker-Barroso E., Heintzmann R., Ng T., Drescher U. (2006). Silencing of EphA3 through a cis interaction with ephrinA5. Nat. Neurosci. 9, 322-330 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

