In vitro and in vivo radiation sensitization of human tumor cells by a novel checkpoint kinase inhibitor, AZD7762
- PMID: 20233881
- PMCID: PMC2851146
- DOI: 10.1158/1078-0432.CCR-09-3277
In vitro and in vivo radiation sensitization of human tumor cells by a novel checkpoint kinase inhibitor, AZD7762
Abstract
Purpose: Inhibition of checkpoint kinase 1 has been shown to enhance the cytotoxicity of DNA-damaging targeted chemotherapy through cell cycle checkpoint abrogation and impaired DNA damage repair. A novel checkpoint kinase 1/2 inhibitor, AZD7762, was evaluated for potential enhancement of radiosensitivity for human tumor cells in vitro and in vivo xenografts.
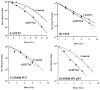
Experimental design: Survival of both p53 wild-type and mutant human cell lines was evaluated by clonogenic assay. Dose modification factors (DMF) were determined from survival curves (ratio of radiation doses for control versus drug treated at 10% survival). Flow cytometry, Western blot, and radiation-induced tumor regrowth delay assays were conducted.
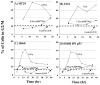
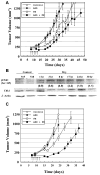
Results: AZD7762 treatment enhanced the radiosensitivity of p53-mutated tumor cell lines (DMFs ranging from 1.6-1.7) to a greater extent than for p53 wild-type tumor lines (DMFs ranging from 1.1-1.2). AZD7762 treatment alone exhibited little cytotoxicity to any of the cell lines and did not enhance the radiosensitivity of normal human fibroblasts (1522). AZD7762 treatment abrogated radiation-induced G(2) delay, inhibited radiation damage repair (assessed by gamma-H2AX), and suppressed radiation-induced cyclin B expression. HT29 xenografts exposed to five daily radiation fractions and to two daily AZD7762 doses exhibited significant radiation enhancement compared with radiation alone.
Conclusions: AZD7762 effectively enhanced the radiosensitivity of mutated p53 tumor cell lines and HT29 xenografts and was without untoward toxicity when administered alone or in combination with radiation. The results of this study support combining AZD7762 with radiation in clinical trials.
Copyright 2010 AACR.
Figures


Similar articles
-
Mechanism of radiosensitization by the Chk1/2 inhibitor AZD7762 involves abrogation of the G2 checkpoint and inhibition of homologous recombinational DNA repair.Cancer Res. 2010 Jun 15;70(12):4972-81. doi: 10.1158/0008-5472.CAN-09-3573. Epub 2010 May 25. Cancer Res. 2010. PMID: 20501833 Free PMC article.
-
The Chk1 inhibitor AZD7762 sensitises p53 mutant breast cancer cells to radiation in vitro and in vivo.Mol Med Rep. 2012 Oct;6(4):897-903. doi: 10.3892/mmr.2012.999. Epub 2012 Jul 20. Mol Med Rep. 2012. PMID: 22825736
-
AZD7762, a novel checkpoint kinase inhibitor, drives checkpoint abrogation and potentiates DNA-targeted therapies.Mol Cancer Ther. 2008 Sep;7(9):2955-66. doi: 10.1158/1535-7163.MCT-08-0492. Mol Cancer Ther. 2008. PMID: 18790776
-
Checkpoint kinase inhibitor synergizes with DNA-damaging agents in G1 checkpoint-defective neuroblastoma.Int J Cancer. 2011 Oct 15;129(8):1953-62. doi: 10.1002/ijc.25842. Epub 2011 Mar 8. Int J Cancer. 2011. PMID: 21154747
-
Improving the Predictive Value of Preclinical Studies in Support of Radiotherapy Clinical Trials.Clin Cancer Res. 2016 Jul 1;22(13):3138-47. doi: 10.1158/1078-0432.CCR-16-0069. Epub 2016 May 6. Clin Cancer Res. 2016. PMID: 27154913 Free PMC article. Review.
Cited by
-
Therapeutic targeting of Chk1 in NSCLC stem cells during chemotherapy.Cell Death Differ. 2012 May;19(5):768-78. doi: 10.1038/cdd.2011.170. Epub 2011 Nov 25. Cell Death Differ. 2012. PMID: 22117197 Free PMC article.
-
CHK1 expression in Gastric Cancer is modulated by p53 and RB1/E2F1: implications in chemo/radiotherapy response.Sci Rep. 2016 Feb 12;6:21519. doi: 10.1038/srep21519. Sci Rep. 2016. PMID: 26867682 Free PMC article.
-
Sensitization of Pancreatic Cancers to Gemcitabine Chemoradiation by WEE1 Kinase Inhibition Depends on Homologous Recombination Repair.Neoplasia. 2015 Oct;17(10):757-66. doi: 10.1016/j.neo.2015.09.006. Neoplasia. 2015. PMID: 26585231 Free PMC article.
-
Irradiation induces glioblastoma cell senescence and senescence-associated secretory phenotype.Tumour Biol. 2016 May;37(5):5857-67. doi: 10.1007/s13277-015-4439-2. Epub 2015 Nov 19. Tumour Biol. 2016. PMID: 26586398
-
ATR/CHK1 inhibitors and cancer therapy.Radiother Oncol. 2018 Mar;126(3):450-464. doi: 10.1016/j.radonc.2017.09.043. Epub 2017 Oct 18. Radiother Oncol. 2018. PMID: 29054375 Free PMC article. Review.
References
-
- Jeggo PA, Lobrich M. Contribution of DNA repair and cell cycle checkpoint arrest to the maintenance of genomic stability. DNA Repair (Amst) 2006;5:1192–8. - PubMed
-
- Ashwell S, Zabludoff S. DNA damage detection and repair pathways--recent advances with inhibitors of checkpoint kinases in cancer therapy. Clin Cancer Res. 2008;14:4032–7. - PubMed
-
- Ashwell S, Janetka JW, Zabludoff S. Keeping checkpoint kinases in line: new selective inhibitors in clinical trials. Expert Opin Investig Drugs. 2008;17:1331–40. - PubMed
-
- Lobrich M, Jeggo PA. The impact of a negligent G2/M checkpoint on genomic instability and cancer induction. Nat Rev Cancer. 2007;7:861–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

