CwpFM (EntFM) is a Bacillus cereus potential cell wall peptidase implicated in adhesion, biofilm formation, and virulence
- PMID: 20233921
- PMCID: PMC2863573
- DOI: 10.1128/JB.01315-09
CwpFM (EntFM) is a Bacillus cereus potential cell wall peptidase implicated in adhesion, biofilm formation, and virulence
Abstract
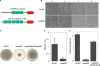
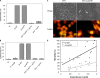
Bacillus cereus EntFM displays an NlpC/P60 domain, characteristic of cell wall peptidases. The protein is involved in bacterial shape, motility, adhesion to epithelial cells, biofilm formation, vacuolization of macrophages, and virulence. These data provide new information on this, so far, poorly studied toxin and suggest that this protein is a cell wall peptidase, which we propose to rename CwpFM.
Figures
Similar articles
-
Structural Modeling of Cell Wall Peptidase CwpFM (EntFM) Reveals Distinct Intrinsically Disordered Extensions Specific to Pathogenic Bacillus cereus Strains.Toxins (Basel). 2020 Sep 14;12(9):593. doi: 10.3390/toxins12090593. Toxins (Basel). 2020. PMID: 32937845 Free PMC article.
-
Characterization of the codY gene and its influence on biofilm formation in Bacillus cereus.Arch Microbiol. 2008 Jun;189(6):557-68. doi: 10.1007/s00203-008-0348-8. Epub 2008 Jan 24. Arch Microbiol. 2008. PMID: 18214442
-
Mechanisms of Bacillus cereus biofilm formation: an investigation of the physicochemical characteristics of cell surfaces and extracellular proteins.Appl Microbiol Biotechnol. 2011 Feb;89(4):1161-75. doi: 10.1007/s00253-010-2919-2. Epub 2010 Oct 9. Appl Microbiol Biotechnol. 2011. PMID: 20936277
-
Epidemiology and pathogenesis of Bacillus cereus infections.Microbes Infect. 2000 Feb;2(2):189-98. doi: 10.1016/s1286-4579(00)00269-0. Microbes Infect. 2000. PMID: 10742691 Review.
-
The multiple roles of the NlpC_P60 peptidase family in mycobacteria - an underexplored target for antimicrobial drug discovery.FEBS Lett. 2025 May;599(9):1203-1221. doi: 10.1002/1873-3468.70021. Epub 2025 Mar 3. FEBS Lett. 2025. PMID: 40028658 Free PMC article. Review.
Cited by
-
The pore-forming haemolysins of bacillus cereus: a review.Toxins (Basel). 2013 Jun 7;5(6):1119-39. doi: 10.3390/toxins5061119. Toxins (Basel). 2013. PMID: 23748204 Free PMC article. Review.
-
Transcriptional regulation and characteristics of a novel N-acetylmuramoyl-L-alanine amidase gene involved in Bacillus thuringiensis mother cell lysis.J Bacteriol. 2013 Jun;195(12):2887-97. doi: 10.1128/JB.00112-13. Epub 2013 Apr 19. J Bacteriol. 2013. PMID: 23603740 Free PMC article.
-
The Bacillus cereus Group: Bacillus Species with Pathogenic Potential.Microbiol Spectr. 2019 May;7(3):10.1128/microbiolspec.gpp3-0032-2018. doi: 10.1128/microbiolspec.GPP3-0032-2018. Microbiol Spectr. 2019. PMID: 31111815 Free PMC article. Review.
-
Advanced Methods for Detection of Bacillus cereus and Its Pathogenic Factors.Sensors (Basel). 2020 May 7;20(9):2667. doi: 10.3390/s20092667. Sensors (Basel). 2020. PMID: 32392794 Free PMC article. Review.
-
Trypan blue dye enters viable cells incubated with the pore-forming toxin HlyII of Bacillus cereus.PLoS One. 2011;6(9):e22876. doi: 10.1371/journal.pone.0022876. Epub 2011 Sep 6. PLoS One. 2011. PMID: 21909398 Free PMC article.
References
-
- Agaisse, H., M. Gominet, O. A. Økstad, A. B. Kolstø, and D. Lereclus. 1999. PlcR is a pleiotropic regulator of extracellular virulence factor gene expression in Bacillus thuringiensis. Mol. Microbiol. 32:1043-1053. - PubMed
-
- Andersson, A., P. E. Granum, and U. Ronner. 1998. The adhesion of Bacillus cereus spores to epithelial cells might be an additional virulence mechanism. Int. J. Food Microbiol. 39:93-99. - PubMed
-
- Andreeva, Z., V. Nesterenko, I. Yurkov, Z. I. Budarina, E. Sineva, and A. S. Solonin. 2006. Purification and cytotoxic properties of Bacillus cereus hemolysin II. Protein Express. Purif. 47:186-193. - PubMed
-
- Arnaout, M., R. Tamburro, S. Bodner, J. Sandlund, G. Rivera, and C. Pui. 1999. Bacillus cereus causing fulminant sepsis and hemolysis in two patients with acute leukemia. J. Pediatr. Hematol. Oncol. 21:431-435. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

