Genetic and functional analysis of the soluble oxaloacetate decarboxylase from Corynebacterium glutamicum
- PMID: 20233922
- PMCID: PMC2863558
- DOI: 10.1128/JB.01678-09
Genetic and functional analysis of the soluble oxaloacetate decarboxylase from Corynebacterium glutamicum
Abstract
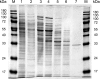
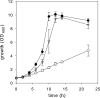
Soluble, divalent cation-dependent oxaloacetate decarboxylases (ODx) catalyze the irreversible decarboxylation of oxaloacetate to pyruvate and CO(2). Although these enzymes have been characterized in different microorganisms, the genes that encode them have not been identified, and their functions have been only poorly analyzed so far. In this study, we purified a soluble ODx from wild-type C. glutamicum about 65-fold and used matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) analysis and peptide mass fingerprinting for identification of the corresponding odx gene. Inactivation and overexpression of odx led to an absence of ODx activity and to a 30-fold increase in ODx specific activity, respectively; these findings unequivocally confirmed that this gene encodes a soluble ODx. Transcriptional analysis of odx indicated that there is a leaderless transcript that is organized in an operon together with a putative S-adenosylmethionine-dependent methyltransferase gene. Biochemical analysis of ODx revealed that the molecular mass of the native enzyme is about 62 +/- 1 kDa and that the enzyme is composed of two approximately 29-kDa homodimeric subunits and has a K(m) for oxaloacetate of 1.4 mM and a V(max) of 201 micromol of oxaloacetate converted per min per mg of protein, resulting in a k(cat) of 104 s(-1). Introduction of plasmid-borne odx into a pyruvate kinase-deficient C. glutamicum strain restored growth of this mutant on acetate, indicating that a high level of ODx activity redirects the carbon flux from oxaloacetate to pyruvate in vivo. Consistently, overexpression of the odx gene in an L-lysine-producing strain of C. glutamicum led to accumulation of less L-lysine. However, inactivation of the odx gene did not improve L-lysine production under the conditions tested.
Figures


References
-
- Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. - PubMed
-
- Birnboim, H. C. 1983. A rapid alkaline extraction method for the isolation of plasmid DNA. Methods Enzymol. 100:243-255. - PubMed
-
- Blombach, B., M. E. Schreiner, M. Moch, M. Oldiges, and B. J. Eikmanns. 2007. Effect of pyruvate dehydrogenase complex deficiency on l-lysine production with Corynebacterium glutamicum. Appl. Microbiol. Biotechnol. 76:615-623. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

