A systemic network for Chlamydia pneumoniae entry into human cells
- PMID: 20233927
- PMCID: PMC2876499
- DOI: 10.1128/JB.01462-09
A systemic network for Chlamydia pneumoniae entry into human cells
Abstract
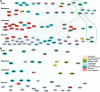
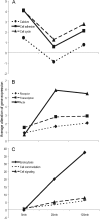
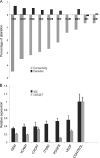
Bacterial entry is a multistep process triggering a complex network, yet the molecular complexity of this network remains largely unsolved. By employing a systems biology approach, we reveal a systemic bacterial-entry network initiated by Chlamydia pneumoniae, a widespread opportunistic pathogen. The network consists of nine functional modules (i.e., groups of proteins) associated with various cellular functions, including receptor systems, cell adhesion, transcription, and endocytosis. The peak levels of gene expression for these modules change rapidly during C. pneumoniae entry, with cell adhesion occurring at 5 min postinfection, receptor and actin activity at 25 min, and endocytosis at 2 h. A total of six membrane proteins (chemokine C-X-C motif receptor 7 [CXCR7], integrin beta 2 [ITGB2], platelet-derived growth factor beta polypeptide [PDGFB], vascular endothelial growth factor [VEGF], vascular cell adhesion molecule 1 [VCAM1], and GTP binding protein overexpressed in skeletal muscle [GEM]) play a key role during C. pneumoniae entry, but none alone is essential to prevent entry. The combination knockdown of three genes (coding for CXCR7, ITGB2, and PDGFB) significantly inhibits C. pneumoniae entry, but the entire network is resistant to the six-gene depletion, indicating a resilient network. Our results reveal a complex network for C. pneumoniae entry involving at least six key proteins.
Figures

Similar articles
-
Chlamydia pneumoniae infection of aortic smooth muscle cells reduces platelet-derived growth factor receptor-beta expression.FEMS Immunol Med Microbiol. 2007 Nov;51(2):363-71. doi: 10.1111/j.1574-695X.2007.00312.x. Epub 2007 Aug 29. FEMS Immunol Med Microbiol. 2007. PMID: 17727656
-
cDNA array analysis of altered gene expression in human endothelial cells in response to Chlamydia pneumoniae infection.Infect Immun. 2001 Mar;69(3):1420-7. doi: 10.1128/IAI.69.3.1420-1427.2001. Infect Immun. 2001. PMID: 11179307 Free PMC article.
-
Chlamydia pneumoniae infection of endothelial cells induces transcriptional activation of platelet-derived growth factor-B: a potential link to intimal thickening in a rabbit model of atherosclerosis.J Infect Dis. 2002 Jun 1;185(11):1621-30. doi: 10.1086/340415. Epub 2002 May 9. J Infect Dis. 2002. PMID: 12023768
-
Apolipoprotein E4 enhances attachment of Chlamydophila (Chlamydia) pneumoniae elementary bodies to host cells.Microb Pathog. 2008 Apr;44(4):279-85. doi: 10.1016/j.micpath.2007.10.002. Epub 2007 Oct 18. Microb Pathog. 2008. PMID: 17997273
-
Interactions of Chlamydia pneumoniae with human endothelial cells.J Infect Dis. 2000 Jun;181 Suppl 3:S479-82. doi: 10.1086/315620. J Infect Dis. 2000. PMID: 10839743 Review.
Cited by
-
Alzheimer's Disease: APP, Gamma Secretase, APOE, CLU, CR1, PICALM, ABCA7, BIN1, CD2AP, CD33, EPHA1, and MS4A2, and Their Relationships with Herpes Simplex, C. Pneumoniae, Other Suspect Pathogens, and the Immune System.Int J Alzheimers Dis. 2011;2011:501862. doi: 10.4061/2011/501862. Epub 2011 Dec 29. Int J Alzheimers Dis. 2011. PMID: 22254144 Free PMC article.
-
Integrated microRNA-mRNA-analysis of human monocyte derived macrophages upon Mycobacterium avium subsp. hominissuis infection.PLoS One. 2011;6(5):e20258. doi: 10.1371/journal.pone.0020258. Epub 2011 May 24. PLoS One. 2011. PMID: 21629653 Free PMC article.
-
Simultaneous transcriptional profiling of bacteria and their host cells.PLoS One. 2013 Dec 4;8(12):e80597. doi: 10.1371/journal.pone.0080597. eCollection 2013. PLoS One. 2013. PMID: 24324615 Free PMC article.
-
Functional modules distinguish human induced pluripotent stem cells from embryonic stem cells.Stem Cells Dev. 2011 Nov;20(11):1937-50. doi: 10.1089/scd.2010.0574. Epub 2011 Jun 20. Stem Cells Dev. 2011. PMID: 21542696 Free PMC article.
-
3-O Sulfated Heparan Sulfate (G2) Peptide Ligand Impairs the Infectivity of Chlamydia muridarum.Biomolecules. 2025 Jul 12;15(7):999. doi: 10.3390/biom15070999. Biomolecules. 2025. PMID: 40723871 Free PMC article.
References
-
- Akira, S., S. Uematsu, and O. Takeuchi. 2006. Pathogen recognition and innate immunity. Cell 124:783-801. - PubMed
-
- Arthos, J., C. Cicala, E. Martinelli, K. Macleod, D. Van Ryk, D. Wei, Z. Xiao, T. D. Veenstra, T. P. Conrad, R. A. Lempicki, S. McLaughlin, M. Pascuccio, R. Gopaul, J. McNally, C. C. Cruz, N. Censoplano, E. Chung, K. N. Reitano, S. Kottilil, D. J. Goode, and A. S. Fauci. 2008. HIV-1 envelope protein binds to and signals through integrin alpha4beta7, the gut mucosal homing receptor for peripheral T cells. Nat. Immunol. 9:301-309. - PubMed
-
- Balana, M. E., F. Niedergang, A. Subtil, A. Alcover, P. Chavrier, and A. Dautry-Varsat. 2005. ARF6 GTPase controls bacterial invasion by actin remodelling. J. Cell Sci. 118:2201-2210. - PubMed
-
- Barabasi, A. L., and Z. N. Oltvai. 2004. Network biology: understanding the cell's functional organization. Nat. Rev. Genet. 5:101-113. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

