NK cells mediate reduction of GVHD by inhibiting activated, alloreactive T cells while retaining GVT effects
- PMID: 20233969
- PMCID: PMC2879101
- DOI: 10.1182/blood-2009-05-222190
NK cells mediate reduction of GVHD by inhibiting activated, alloreactive T cells while retaining GVT effects
Abstract
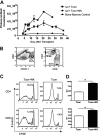
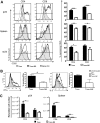
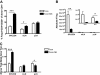
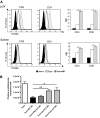
Natural killer (NK) cells suppress graft-versus-host disease (GVHD) without causing GVHD themselves. Our previous studies demonstrated that allogeneic T cells and NK cells traffic similarly after allogeneic bone marrow transplantation (BMT). We therefore investigated the impact of donor NK cells on donor alloreactive T cells in GVHD induction. Animals receiving donor NK and T cells showed improved survival and decreased GVHD score compared with controls receiving donor T cells alone. Donor T cells exhibited less proliferation, lower CD25 expression, and decreased interferon-gamma (IFN-gamma) production in the presence of NK cells. In vivo, we observed perforin- and Fas ligand (FasL)-mediated reduction of donor T cell proliferation and increased T cell apoptosis in the presence of NK cells. Further, activated NK cells mediated direct lysis of reisolated GVHD-inducing T cells in vitro. The graft-versus-tumor (GVT) effect was retained in the presence of donor NK cells. We demonstrate a novel mechanism of NK cell-mediated GVHD reduction whereby donor NK cells inhibit and lyse autologous donor T cells activated during the initiation of GVHD.
Figures


References
-
- Copelan EA. Hematopoietic stem-cell transplantation. N Engl J Med. 2006;354(17):1813–1826. - PubMed
-
- Ferrara JL, Deeg HJ. Graft-versus-host disease. N Engl J Med. 1991;324(10):667–674. - PubMed
-
- Yokoyama WM, Kim S, French AR. The dynamic life of natural killer cells. Annu Rev Immunol. 2004;22:405–429. - PubMed
-
- Ruggeri L, Capanni M, Urbani E, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295(5562):2097–2100. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

