The pan-HDAC inhibitor vorinostat potentiates the activity of the proteasome inhibitor carfilzomib in human DLBCL cells in vitro and in vivo
- PMID: 20233973
- PMCID: PMC2881506
- DOI: 10.1182/blood-2009-12-257261
The pan-HDAC inhibitor vorinostat potentiates the activity of the proteasome inhibitor carfilzomib in human DLBCL cells in vitro and in vivo
Retraction in
-
Dasmahapatra G, Lembersky D, Kramer L, Fisher RI, Friedberg J, Dent P, Grant S. The pan-HDAC inhibitor vorinostat potentiates the activity of the proteasome inhibitor carfilzomib in human DLBCL cells in vitro and in vivo. Blood. 2010;115(22):4478-4487.Blood. 2019 Jul 4;134(1):95. doi: 10.1182/blood-2019-05-901223. Epub 2019 May 24. Blood. 2019. PMID: 31126919 Free PMC article. No abstract available.
Abstract
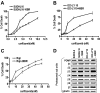
Interactions between histone deacetylase inhibitors (HDACIs) and the novel proteasome inhibitor carfilzomib (CFZ) were investigated in GC- and activated B-cell-like diffuse large B-cell lymphoma (ABC-DLBCL) cells. Coadministration of subtoxic or minimally toxic concentrations of CFZ) with marginally lethal concentrations of HDACIs (vorinostat, SNDX-275, or SBHA) synergistically increased mitochondrial injury, caspase activation, and apoptosis in both GC- and ABC-DLBCL cells. These events were associated with Jun NH2-terminal kinase (JNK) and p38MAPK activation, abrogation of HDACI-mediated nuclear factor-kappaB activation, AKT inactivation, Ku70 acetylation, and induction of gammaH2A.X. Genetic or pharmacologic JNK inhibition significantly diminished CFZ/vorinostat lethality. CFZ/vorinostat induced pronounced lethality in 3 primary DLBCL specimens but minimally affected normal CD34(+) hematopoietic cells. Bortezomib-resistant GC (SUDHL16) and ABC (OCI-LY10) cells exhibited partial cross-resistance to CFZ. However, CFZ/vorinostat dramatically induced resistant cell apoptosis, accompanied by increased JNK activation and gammaH2A.X expression. Finally, subeffective vorinostat doses markedly increased CFZ-mediated tumor growth suppression and apoptosis in a murine xenograft OCI-LY10 model. These findings indicate that HDACIs increase CFZ activity in GC- and ABC-DLBCL cells sensitive or resistant to bortezomib through a JNK-dependent mechanism in association with DNA damage and inhibition of nuclear factor-kappaB activation. Together, they support further investigation of strategies combining CFZ and HDACIs in DLBCL.
Figures
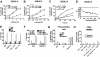

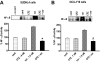
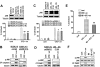

Comment in
-
Findings of Research Misconduct.NIH Guide Grants Contracts (Bethesda). 2015 Dec 18:NOT-OD-16-040. NIH Guide Grants Contracts (Bethesda). 2015. PMID: 26693581 Free PMC article. No abstract available.
-
Findings of Research Misconduct.Fed Regist. 2015 Dec 10;80(237):76703-76704. Fed Regist. 2015. PMID: 27737268 Free PMC article. No abstract available.
References
-
- Coiffier B, Lepage E, Briere J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346(4):235–242. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

