Rationale and design of the Pharmacist Intervention for Low Literacy in Cardiovascular Disease (PILL-CVD) study
- PMID: 20233982
- PMCID: PMC3021350
- DOI: 10.1161/CIRCOUTCOMES.109.921833
Rationale and design of the Pharmacist Intervention for Low Literacy in Cardiovascular Disease (PILL-CVD) study
Abstract
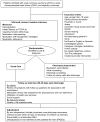
Background: Medication errors and adverse drug events are common after hospital discharge due to changes in medication regimens, suboptimal discharge instructions, and prolonged time to follow-up. Pharmacist-based interventions may be effective in promoting the safe and effective use of medications, especially among high-risk patients such as those with low health literacy.
Methods and results: The Pharmacist Intervention for Low Literacy in Cardiovascular Disease (PILL-CVD) study is a randomized controlled trial conducted at 2 academic centers-Vanderbilt University Hospital and Brigham and Women's Hospital. Patients admitted with acute coronary syndrome or acute decompensated heart failure were randomly assigned to usual care or intervention. The intervention consisted of pharmacist-assisted medication reconciliation, inpatient pharmacist counseling, low-literacy adherence aids, and tailored telephone follow-up after discharge. The primary outcome is the occurrence of serious medication errors in the first 30 days after hospital discharge. Secondary outcomes are health care utilization, disease-specific quality of life, and cost-effectiveness. Enrollment was completed September 2009. A total of 862 patients were enrolled, and 430 patients were randomly assigned to receive the intervention. Analyses will determine whether the intervention was effective in reducing serious medication errors, particularly in patients with low health literacy.
Conclusions: The PILL-CVD study was designed to reduce serious medication errors after hospitalization through a pharmacist-based intervention. The intervention, if effective, will inform health care facilities on the use of pharmacist-assisted medication reconciliation, inpatient counseling, low-literacy adherence aids, and patient follow-up after discharge. Clinical Trial Registration- clinicaltrials.gov. Identifier: NCT00632021.
Figures
References
-
- Coleman EA, Berenson RA. Lost in transition: challenges and opportunities for improving the quality of transitional care. Ann Intern Med. 2004;141:533–536. - PubMed
-
- Coleman EA, Smith JD, Raha D, Min SJ. Posthospital medication discrepancies: prevalence and contributing factors. Arch Intern Med. 2005;165:1842–1847. - PubMed
-
- Clark PA, Drain M, Gesell SB, Mylod DM, Kaldenberg DO, Hamilton J. Patient perceptions of quality in discharge instruction. Patient Educ Couns. 2005;59:56–68. - PubMed
-
- Forster AJ, Murff HJ, Peterson JF, Gandhi TK, Bates DW. The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138:161–167. - PubMed
-
- Bates DW, Cullen DJ, Laird N, Petersen LA, Small SD, Servi D, Laffel G, Sweitzer BJ, Shea BF, Hallisey R, Vander Vliet M, Nemeskal R, Leape L. Incidence of adverse drug events and potential adverse drug events. Implications for prevention. ADE Prevention Study Group. JAMA. 1995;274:29–34. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical