CNS-targeted gene therapy improves survival and motor function in a mouse model of spinal muscular atrophy
- PMID: 20234094
- PMCID: PMC2846065
- DOI: 10.1172/JCI41615
CNS-targeted gene therapy improves survival and motor function in a mouse model of spinal muscular atrophy
Abstract
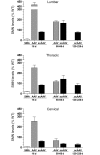
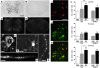
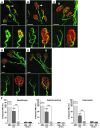
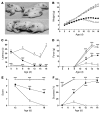
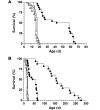
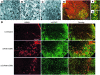
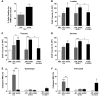
Spinal muscular atrophy (SMA) is a neuromuscular disease caused by a deficiency of survival motor neuron (SMN) due to mutations in the SMN1 gene. In this study, an adeno-associated virus (AAV) vector expressing human SMN (AAV8-hSMN) was injected at birth into the CNS of mice modeling SMA. Western blot analysis showed that these injections resulted in widespread expression of SMN throughout the spinal cord, and this translated into robust improvement in skeletal muscle physiology, including increased myofiber size and improved neuromuscular junction architecture. Treated mice also displayed substantial improvements on behavioral tests of muscle strength, coordination, and locomotion, indicating that the neuromuscular junction was functional. Treatment with AAV8-hSMN increased the median life span of mice with SMA-like disease to 50 days compared with 15 days for untreated controls. Moreover, injecting mice with SMA-like disease with a human SMN-expressing self-complementary AAV vector - a vector that leads to earlier onset of gene expression compared with standard AAV vectors - led to improved efficacy of gene therapy, including a substantial extension in median survival to 157 days. These data indicate that CNS-directed, AAV-mediated SMN augmentation is highly efficacious in addressing both neuronal and muscular pathologies in a severe mouse model of SMA.
Figures